Summary
 Endobronchial valves are one-way valves placed in the airways of the most damaged parts of the lungs using bronchoscopy.
Endobronchial valves are one-way valves placed in the airways of the most damaged parts of the lungs using bronchoscopy.- Endobronchial valves may be used as an alternative to surgery to achieve lung volume reduction and improve breathing in some people with severe emphysema.
- Careful patient selection is critical to ensure that only the subset of patients who may benefit undergo the procedure.
- Limited longer-term (up to three years) evidence suggests that some improvements in lung function after valve placement decline over time – perhaps due to disease progression.
- The focus of this bulletin is on two endobronchial valve technologies:
- the Zephyr Endobronchial Valve System (Pulmonx Corporation)
- the Spiration Valve System (Spiration, Inc./Olympus).
- This bulletin builds on a 2019 CADTH Rapid Response Report that critically appraised recent studies on the clinical effectiveness of endobronchial valves.
Methods
CADTH Horizon Scanning bulletins present an overview of the technology and available evidence. They are not systematic reviews and do not involve the critical appraisal of all studies or include a detailed summary of study findings. They are not intended to provide recommendations for or against a particular technology. A 2019 CADTH Rapid Response Report, developed to support this bulletin, includes a detailed critical appraisal of the quality of recent evidence.
Literature Search Strategy
A limited literature search was conducted by an information specialist on key resources including PubMed, the Cochrane Library, the University of York Centre for Reviews and Dissemination databases, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were endobronchial valves and emphysema. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and October 3, 2019. A monthly alert in PubMed, using a modified search strategy, updated the literature search until February 2020.
Study Selection
One author screened the literature search results and reviewed the full text of all potentially relevant studies. Studies were considered for inclusion if the intervention was the use of EBVs for the treatment of emphysema. Conference abstracts and grey literature were only included if they provided additional information to that available in the published studies.
Peer Review
A draft version of this bulletin was reviewed by one clinical expert. The manufacturers were also given the opportunity to provide information and comment on an earlier draft. One manufacturer submitted comments.
Background
Emphysema is a form of chronic obstructive pulmonary disease (COPD) — a major cause of morbidity and mortality in Canada and worldwide.1,2 As COPD is more prevalent in the elderly, the health care costs associated with management of this condition are expected to increase due to the aging Canadian population.3 Health care costs for Canadians with COPD are approximately $6,300 per year higher than for those without the disease — mainly due to increased hospitalizations.3-5
As with other types of COPD, emphysema is progressive and irreversible.6,7 The main causes of emphysema are cigarette smoking and long-term occupational or environmental exposure to toxic gases and air pollutants.8 Some individuals with a genetic risk factor (alpha1-antitrypsin deficiency) are also at higher risk for developing the condition.8
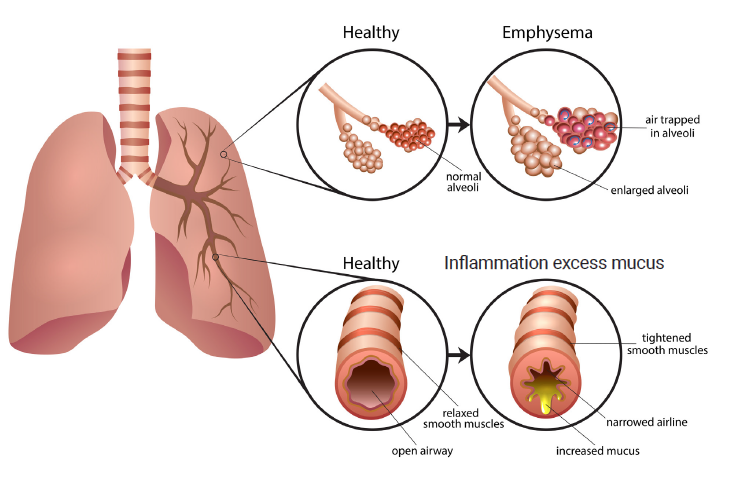
Emphysema manifests as damage to walls between the air sacs (alveoli), allowing larger pockets of air to form in the lungs and leading to loss of the lung’s elasticity and natural recoil. This results in difficulty exchanging oxygen for carbon dioxide, hyperinflation of the lungs, and expiratory collapse of the small airways, making breathing more difficult. Emphysema may affect some parts of the lungs more than others such that the hyperinflated areas can compress on and affect the function of the less damaged areas (Dr. Dominic Carney, University of Alberta, Edmonton, AB: personal communication, Feb 12, 2020).
In addition to shortness of breath (dyspnea), people with emphysema have chronic cough and fatigue, and are more susceptible to respiratory infections.9 As emphysema progresses, even with optimal treatment, difficulty breathing and hypoxemia limit the ability to carry out daily activities, which worsens muscle weakness, depression, anxiety, and overall quality of life.10
Endobronchial valves are one-way valves that are placed in the airways in the most damaged areas of the lung via a flexible bronchoscope with a deployment catheter and loader.6 During inhalation, the valves close, blocking air flow into the diseased lobe of the lung. During exhalation, the valves open and trapped air in the diseased lobe escapes through the valves until the lung volume of the treated lobe is reduced.6,11 The procedure is sometimes called bronchoscopic or endoscopic lung volume reduction, as the valves cause atelectasis (deflation of a lobe of the lung) to reduce the size of the damaged lung without requiring surgery.
Not everyone with severe emphysema will benefit from endobronchial valves — in particular, those with collateral ventilation are unlikely to benefit.12,13 Collateral ventilation is airflow from accessory pathways (rather than the main airways) into the damaged lobe of the lung that is targeted for valve treatment. If collateral ventilation is present, even with endobronchial valves placed in the main airways, air will enter through the collateral pathways, preventing the hyperinflated lobe of the lung from deflating as intended (Dr. Dominic Carney: personal communication, Feb 12, 2020).
The Technology
Various lung volume reduction techniques have been investigated since the early surgical trials in the 1950s.14 In addition to surgical lung volume reduction procedures, less invasive endobronchial procedures have been studied, including implantable valves, coils, and stents, as well as thermal water vapour ablation and sealants.12,14 Overall, these procedures have shown benefits in lung function, exercise capacity, and quality of life for some patients — but with increased complications and post-procedure illnesses.2,8,14 This bulletin focuses on two commercially available endobronchial valves, the Zephyr Endobronchial Valve System and the Spiration Valve.
Zephyr Endobronchial Valve System (Pulmonx Corporation)
The Zephyr endobronchial valve (EBV) is a one-way valve, approximately the size of a pencil eraser, with a self-expanding nitinol (nickel-titanium) scaffold covered by a silicone membrane.11 The valves are intended to be left in place permanently but can be removed if necessary.11 The Zephyr valve is implanted using a Zephyr Endobronchial Delivery Catheter inserted through a bronchoscope to the damaged lobe of the lung. Multiple valves are usually needed to block airflow into the target area.11
Pulmonx has developed the proprietary Chartis System and StratX lung analysis platform to measure airflow and assess collateral ventilation. These tools are intended to enable optimal patient selection and treatment planning.11,15,16
The Zephyr EBV procedure takes from 30 to 60 minutes and is performed in the hospital with the patient receiving sedation or general anesthesia.11,17 The manufacturer estimates that one in five patients may require a subsequent procedure to adjust or remove valves, or to insert additional valves.11
Spiration Valve System (Spiration, Inc. Olympus)
The Spiration Valve System consists of a delivery device and an implantable, one-way EBV. The valve is intended to reduce over-inflation in a target area of the damaged lung for the treatment of severe emphysema.18 Similar to a tiny umbrella, the valve has a nitinol frame with a polyurethane membrane and five anchors to secure the valve.19 The valve is inserted into the lung through a bronchoscope. Although the valves are intended to be left in place permanently, they can be extracted with a removal device included in the system.18
In the EMPROVE study, the Spiration valve procedure took approximately 30 minutes, and the procedure was performed in a hospital, with the patient receiving sedation or general anesthesia.6 Of the 113 study participants who received valves, 26 (23%) needed subsequent bronchoscopies to remove or replace valves.20
Availability
The Zephyr Endobronchial Valve System (formerly the Emphasys valve) does not currently have a Health Canada licence, but it received US FDA approval in 2018.21 The timing for licensing in Canada is not known.
The Spiration Valve System (Spiration/Olympus Medical) received a Class III medical device licence from Health Canada in 2016.21,22 The Spiration Valve System received US FDA approval in 2018.21 (Spiration’s earlier IBV Valve System initially received FDA Humanitarian Device Exemption in 2008 for the treatment of prolonged air leaks in the lung).23
Cost
EBVs are an add-on therapy that do not replace the standard medical management of patients with emphysema.11
No information on the costs of EBVs was provided by the manufacturers.
Two European cost-effectiveness studies estimated per patient costs of Zephyr EBV placement.24,25 The study from the Netherlands, using 2016 costs, estimated a per patient cost of €13,197 (€1,900 per valve — including an average of 4.5 EBVs, 1.4 endobronchial catheters, and the cost of the Chartis assessment).24 The study from Germany, using 2014 costs, estimated a per patient cost of €9,851: €4,337.47 as the German diagnosis-related group payment for valve placement, with an add-on payment of €1,702.60 for each additional valve, and an average of 3.08 valves per patient.25
Multiple valves are typically needed to achieve occlusion of the airflow. In the Zephyr EBV LIBERATE trial, an average of four valves were used for each patient and up to eight Zephyr valves have been used in a procedure.11
Who Might Benefit?
The Public Health Agency of Canada estimates that more than two million Canadians, or 10% of the population older than 35, are living with diagnosed COPD, including chronic bronchitis and emphysema — both of which often affect those with COPD.26-28 The prevalence of COPD is higher in older populations. In addition, many people with mild to moderate emphysema may not yet have been diagnosed with the disease.1,26
A 2014 Statistics Canada study estimated that approximately 1% of those with COPD had moderate-to-severe COPD, but cautioned that the estimate was subject to “large variation.”1 In addition to increased prevalence in older populations, the prevalence of COPD varies across Canada. Moreover, populations at greater risk for health inequities due to the social determinants of health have an increased risk for developing COPD.8,26
One US study estimated that less than 1% of patients with severe emphysema receive lung volume reduction surgery, but that up to 15% of patients with severe emphysema may be candidates for surgery.29 Other authors have speculated that likely more than 15% of those with severe emphysema could qualify for EBV treatment.30
Germany was an early adopter of EBVs, which have been used in clinical practice there since 2007.31 In a recent German study of the diffusion of endobronchial lung volume reduction procedures, about 5% of people newly diagnosed with severe emphysema each year (1,655 individuals in 2016) received either endobronchial coils or valves.31 An estimated 12,000 coil or valve procedures were performed in Germany up to 2016; 36% of these procedures used coils.31 The authors noted that earlier estimates were that approximately 20% of people with severe emphysema may be candidates for endobronchial lung volume reduction.31
Current Practice
A diagnosis of emphysema considers the individual’s risk factors (history of smoking or occupational exposure to air pollutants), symptoms (dyspnea, chronic cough with sputum production, recurrent respiratory tract infections), and post-bronchodilator airflow limitation as measured by spirometry.8,32
Management of emphysema includes support for smoking cessation, pneumococcal and influenza vaccinations, and pulmonary rehabilitation.32 Pulmonary rehabilitation (psychosocial support, education for patient self-management of the condition, and exercise therapy tailored to the needs of the individual) is recommended as an effective component of standard treatment, but one that, for various reasons, may not be used.7-10,32,33
Drug therapies include short- and long-acting bronchodilators, oral or inhaled corticosteroids, beta agonists, and other drugs used for the management of COPD. Drug therapies alleviate symptoms and exacerbations, and improve exercise tolerance, but they do not stop the progression of the disease.8 Standard treatment may also include supplemental oxygen therapy.32 As many individuals with COPD are older, they often have comorbidities, such as cardiovascular disease, diabetes, lung cancer, or osteoporosis, which must also be managed.8
For some patients who have not received adequate symptom relief despite optimal drug and other therapies, surgical lung volume reduction (excision of the most damaged lung tissue) or lung transplantation may be options.7,10 However, not all patients are eligible for surgical procedures, and the shortage of donor lungs for transplant surgery means that alternative treatment options are needed.10
Summary of the Evidence
Results
The 2019 CADTH Rapid Response report34 included one 2019 systematic review (with studies of both the Zephyr and Spiration valves, covering the literature to July 2018)12 and two subsequent randomized controlled trials of the Spiration valves published in 2019 (the REACH trial35 and the EMPROVE trial6). The systematic review found that the Zephyr EBVs improved lung function, exercise capacity, and quality of life, and reduced dyspnea for up to 12 months. However, the risk of serious adverse events was increased in patients who received EBVs.12,34
The CADTH Rapid Response Report also noted that patients treated with the Spiration Valve System in the two recent randomized studies had significantly improved lung function (forced expiratory volume in one second or FEV1) compared to patients treated with standard medical care. One of the two Spiration valve studies found an improvement in quality of life; this study also found no significant improvement in exercise capacity. The Rapid Response concluded that further evidence of efficacy and safety of the Spiration Valve System is needed.34
The CADTH Rapid Response Report highlighted that the available studies have compared valve treatment to standard medical care, rather than to other interventions, such as lung volume reduction surgery or lung transplantation.34
Other Information
Information published since the CADTH Rapid Response Report includes a 2019 systematic review and meta-analysis of studies of the Spiration Valve System (covering the literature to August 2019)36 and a German retrospective patient database study.37 These publications have not been critically appraised but are subsequently summarized.
The systematic review analyzed four randomized controlled trials with a total of 629 participants (364 in the valve treatment arm and 265 in the control group). Unlike most of the earlier studies, it was not industry funded. The authors found that the subgroup of patients in the two most recent trials, who were assessed prior to valve treatment to ensure a lack of collateral ventilation, had more improvement in lung function. Quality of life and breathlessness also improved in patients who received the Spiration valve; however, no significant benefit in exercise capacity (assessed using the six-minute walk) was found.36
The German patient database study reported up to a three-year follow-up on 256 patients who had had an absence of collateral ventilation confirmed prior to receiving either the Zephyr or Spiration valves.37 At six months (data available for 200 patients), 37% of patients met the efficacy threshold for improvement in FEV1,and both the six-minute walk and Modified Medical Research Council dyspnea scale scores were also improved. At the three-year follow-up (data available for 66 patients), some patients still showed an improvement in some measures of lung function over baseline.37 Better outcomes were seen in patients with more complete atelectasis of the treated lobe. The percentage of patients with an improvement in FEV1 declined — from 74 of 200 patients (37%) at six months to six of 65 patients (9.2%) at three years.37 The investigators concluded that the clinical improvement during the first year subsequently declined gradually — probably due to the progression of COPD. They further commented that, even with EBV intervention, it is important to optimize medical management and exercise therapy to slow the progression of the disease.37
Safety
The CADTH Rapid Response Report and earlier UK NICE‒National Institute for Health and Care Excellence guidance both note that EBV treatment was associated with a significantly higher risk of serious adverse events compared with standard medical care.17,34 Pneumothorax — a leak in the lining of the lung that allows air to escape into the pleural space between the lung and wall of the chest — was the most common complication following EBV procedures.34 The systematic review included in the Rapid Response Report did not calculate rates of pneumothorax separately from other serious adverse events.34
Other Information
The manufacturer’s instructions for use of the Zephyr EBV note that pneumothorax is a common adverse event, particularly within the first three days following the procedure.11 The instructions recommend that patients remain in hospital for at least three nights post-procedure and be given instructions to recognize pneumothorax and obtain emergency care if this occurs after they are discharged.11 The US FDA MAUDE database of adverse events includes several reports of pneumothorax associated with EBV valves.38 (Note that the MAUDE database does not capture all the adverse events that occur with any given device.)38
In the published literature, the incidence of pneumothorax was significantly higher in patients treated with EBVs compared to those receiving medical management, particularly in the short-term period post-procedure.6,39,40 Most patients with pneumothorax required hospital treatment, with chest tube insertion.36,40
The 2019 systematic review on Spiration valves found that acute exacerbations of COPD were also higher in the patients who received EBVs compared to those who received medical management during the first six months post-procedure.36
The need for subsequent bronchoscopy after valve placement has been estimated to occur in 19% to 35% of patients, and valve removal was necessary in 3% to 21% of patients.39 In addition to valve adjustment, reasons for valve removal include “treatment failure,” post-stenotic pneumonia, and hemoptysis (coughing up blood).17,41 One study reported that valve replacement was needed in 17% of patients.42 Valve migration, sometimes due to granulation tissue formation associated with implanted devices, was also identified as a potential complication.42 Although the placement of EBVs is intended to be a reversible procedure, complications can prevent the removal of a valve.41
Another potential adverse event is bacterial colonization. A 2018 German study found increased levels of bacterial colonization in follow-up bronchoscopies at twelve to 24 months after valve implantation.43 Various types of “potentially pathogenic” microorganisms were detected, including Staphylococcus aureus and Pseudomonas aeruginosa, which can cause exacerbations of COPD. The authors noted the need to consider this risk in regular patient follow-up.43
The US FDA is reviewing concerns regarding biological responses to metal in implanted medical devices.44,45 Although EBVs are not specifically mentioned, the long-term monitoring of patients for possible immune reactions may be warranted. A recent case report from Switzerland noted possible nickel hypersensitivity in one patient who received EBVs.46 The authors note that up to 19% of people have a contact allergy to nickel and that their preliminary tests on two new and four used EBVs found nickel release from both, despite the silicone cover.46 The manufacturers’ contraindications for the use of EBVs include patients with known allergies to nickel or titanium.11,18
Cost-Effectiveness
Two cost-effectiveness studies were identified: a 2014 study from Germany25 and a 2018 study from the Netherlands.24 Both studies concluded that EBV treatment was cost-effective compared to standard medical care or other treatment options for appropriately selected patients with severe emphysema.24,25
Concurrent Developments
Other new or emerging endoscopic treatments for severe emphysema include: bronchial thermal vapour ablation (InterVapor, Uptake Medical), polymeric foam lung volume reduction with a biodegradable gel (AeriSeal, Pulmonyx), and targeted radiofrequency lung denervation (dNerva, Nuvaira Inc.).10,47-49 Another development, the AIR-AD device (RightAir), is a wearable vest that provides non-invasive ventilation to reduce hyperventilation and assist breathing.50 The manufacturer is currently applying for US FDA approval of the device.51
In the UK, the CELEB trial is comparing clinical outcomes and costs of lung volume reduction surgery and EBV treatment with up to one-year follow-up.52 Also in the UK, the UK lung volume reduction study registry is tracking patients who received lung volume reduction procedures (including endobronchial valves) from 2016 to 2019.53 In the Netherlands, the SOLVE trial (NCT03474471) is investigating how best to combine pulmonary rehabilitation with EBV treatment (for example, before or after valve treatment or only as an option post-treatment).39 In addition, the development of new treatments to address collateral ventilation could increase the potential patient population for EBVs.39,54
Operational Issues
Clinician Training and Learning Curve
Pulmonx provides a training program for clinicians and states that only trained physicians should use the Zephyr EBV.11 Clinicians in the REACH trial had little experience with EBV placement before the study but few procedural-related adverse events were reported, which may indicate that the procedure is relatively simple for clinicians to perform.35 However, the 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report highlights the risk of pneumothorax following valve placement, and recommends that clinicians performing these procedures have experience in managing the procedure-related complications.8
Dutch investigators recommend that, as in the Netherlands, EBV procedures should only be performed in centres of clinical excellence and that, based on their experience, clinicians should perform at least 15 to 20 procedures per year to maintain their skills.55
Patient Selection
Patients whose lungs have collateral ventilation are unlikely to benefit from EBVs.2,17,48 Careful patient selection and follow-up care are key to achieving the best possible patient outcomes and to managing the increased risk for respiratory adverse events post-procedure.12,48 Outcomes reported in earlier studies, where patients were not assessed for the presence of collateral ventilation, could potentially have been improved through more appropriate patient selection.36,56 Improved ways to predict the risk of pneumothorax post-procedure are also needed.39
The 2017 UK NICE guidance notes that patient eligibility for EBVs should be determined by a multidisciplinary team with experience in treating emphysema. The team should include a respiratory physician (pulmonologist), radiologist, thoracic surgeon, and a respiratory nurse.17 The need for a multidisciplinary team was also stressed by investigators in other countries, some of which included an interventional pulmonologist on the team.39,48,57
The importance of patients receiving pulmonary rehabilitation before being considered for EBV treatment, as well as in subsequent care, has also been noted.6,17,36,57
Authors of a 2018 German paper describing their centre’s clinical experience commented that patients with a six-minute walk test more than 420 metres are not “physically limited enough to notice a clinical improvement” from lung volume reduction treatments.57 The authors also noted that other comorbidities can affect exercise capacity and that lung volume reduction may not be appropriate for these patients.57
Procedural Times, Length of Hospital Stay, Number of Valves, and Valve Removal
Reported procedural times range from approximately 21 minutes to just under an hour.35,58 Sedation has been used in some procedures, but most patients receive general anesthesia.35,58
In the REACH trial of the Spiration Valve System, an average of 5.2 valves were used per patient.35 The average length of hospital stay following the procedure was six days.35 In the EMPROVE trial of the Spiration Valve System,6an average of four valves were used per patient. The average length of hospital stay following the procedure was four days.6 Patient materials provided by Pulmonx for the Zephyr valve note a typical hospital stay of three to five days, unless complications occur. Complications requiring a longer hospital stay may occur in up to one in three procedures.11
Patient Preferences
A modelling study, funded by Pulmonx, conducted a discrete choice experiment in individuals with emphysema to determine patient preferences and risk tolerance for treatment.30 Modelling based on the 294 survey responses and different levels of risk-benefit estimated that 71% of patients would choose a treatment such as EBVs to reduce breathlessness, 6% would choose lung volume reduction surgery, and about 23% would choose to remain with current medical management.30
In a 2019 German study of patients considered for endobronchial therapies for severe emphysema, 21 of 115 patients (18%) of those eligible to receive EBV chose not to undergo the procedure — mainly due to concerns about potential complications.48
Uptake
A study of the adoption of bronchoscopic lung volume reduction (including both valves and coils) in Germany found that there was a slight increase in use during the period 2007 to 2016.31 Based on the German data, the authors estimated the potential annual patient numbers for endobronchial lung volume reduction procedures in other European countries.31 Factors that may influence the uptake of these procedures include: clinical expertise with training in the use of these techniques, differing disease management pathways, reimbursement decisions, and the introduction of competing new endobronchial technologies. Factors that could result in a decline in use include reduction of smoking and environmental pollution, “limited acceptance” of the treatments by referring clinicians, and lack of coverage by health insurers.31
Investigators involved in EBV studies in the Netherlands note that, ideally, the introduction of EBV treatments should be limited to specialized centres with expertise in surgical and bronchoscopic lung procedures.39 Investigators also recommended the creation of national or international patient registries to track outcomes and complications as the treatment is used more often in routine clinical practice.31,39,55 Such registries have been established in Germany, Italy, the Netherlands, and the UK.55
Final Remarks
Patient outcomes may improve with the refinement of methods to assess patient eligibility and identify those at risk for pneumothorax.59 In addition, less invasive methods for surgical lung volume reduction may have reduced the mortality associated with this procedure.59
To fully understand the comparative effectiveness of this treatment, trials that directly compare the two commercially available EBVs and other lung volume reduction techniques are needed. Also needed is further evidence of the durability of EBV treatment from longer follow-up studies.12,19,37,39 Longer-term evidence should include information on functional outcomes, adverse events, and mortality, as well as the number of subsequent bronchoscopies required to check or adjust valve placement, or to remove valves.39 This would provide greater certainty regarding whether the benefits of valve treatment outweigh the risks and costs involved.
References
1. Evans J, Chen Y, Camp PG, Bowie DM, McRae L. Estimating the prevalence of COPD in Canada: Reported diagnosis versus measured airflow obstruction. Health Rep. 2014;25(3):3-11.
2. van Agteren JE, Hnin K, Grosser D, Carson KV, Smith BJ. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;2:Cd012158.
3. Tran DT, Thanh NX, Ohinmaa A, Mayers I, Jacobs P. Current and future direct healthcare cost burden of chronic obstructive pulmonary disease in Alberta, Canada. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 2019:1-9.
4. Khakban A, Sin DD, FitzGerald JM, et al. Ten-year trends in direct costs of COPD: A population-based study. Chest. 2015;148(3):640-646.
5. Gershon AS, Guan J, Victor JC, Goldstein R, To T. Quantifying health services use for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(6):596-601.
6. Criner GJ, Delage A, Voelker K, et al. Improving Lung Function in Severe Heterogenous Emphysema with the Spiration(R) Valve System (EMPROVE): A multicenter, open-label, randomized, controlled trial. Am J Respir Crit Care Med. 2019;200(11):1354-1362.
7. Herth FJF, Slebos DJ, Criner GJ, Valipour A, Sciurba F, Shah PL. Endoscopic lung volume reduction: An Expert Panel recommendation - Update 2019. Respiration. 2019;97(6):548-557.
8. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (GOLD): 2020 report. Fontana (WI): Global Initiative for Chronic Obstructive Lung Disease, Inc. (GOLD); 2020: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed 2020 Jan 29.
9. Izenberg D, Marwaha S. Everyone agrees: pulmonary rehab helps people who have COPD. So why do so few access it? Healthy Debate 2018; https://healthydebate.ca/2018/01/topic/copd-pulmonary-rehab-access-canada. Accessed 2019 Jan 25.
10. Herth FJF, Slebos DJ, Shah PL, et al. Protocol of a randomized controlled study of the PneumRx Endobronchial Coil System versus standard-of-care medical management in the treatment of subjects with severe emphysema (ELEVATE). Respiration. 2019;98(6):512-520.
11. Zephyr Endobronchial Valve System: instructions for use. Redwood City (CA): Pulmonx Corporation; 2018: https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180002C.pdf. Accessed 2020 Jan 10.
12. Rustagi N, Singh S, Dutt N, et al. Efficacy and safety of stent, valves, vapour ablation, coils and sealant therapies in advanced emphysema: A meta-analysis. Turk Thorac J. 2019;20(1):43-60.
13. Van Der Molen MC, Klooster K, Hartman JE, Slebos DJ. Lung volume reduction with endobronchial valves in patients with emphysema. Expert Rev Med Devices. 2018;15(11):847-857.
14. Marruchella A, Faverio P, Bonaiti G, Pesci A. History of lung volume reduction procedures. J Thorac Dis. 2018;10(Suppl 27):S3326-s3334.
15. Pulmonx. The Chartis® Tablet. 2020; https://pulmonx.com/chartis/. Accessed 2020 Mar 5.
16. Pulmonx. StratX® lung analysis platform. 2020; https://pulmonx.com/stratx/. Accessed 2020 Mar 5.
17. National Institute for Health and Care Excellence. Endobronchial valve insertion to reduce lung volume in emphysema. (Interventional procedures guidance IPG600) 2017; https://www.nice.org.uk/guidance/ipg600. Accessed 2019 Dec 12.
18. U.S. Food and Drug Administration. Spiration Valve® System - P180007. 2019; https://www.fda.gov/medical-devices/recently-approved-devices/spiration-valver-system-p180007. Accessed 2019 Dec 6.
19. Hopkinson NS. Lung volume reduction: Apex treatments and the ecology of chronic obstructive pulmonary disease care. Am J Respir Crit Care Med. 2019;200(11):1329-1331.
20. Summary of safety and effectiveness data (SSED): [Spiration Valve System]. Silver Spring (MD): U.S. Food and Drug Administration; 2018: https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180007B.pdf. Accessed 2020 Feb 20.
21. U.S. Food and Drug Administration. Zephyr® Endobronchial Valve System - P180002. 2018; https://www.fda.gov/medical-devices/recently-approved-devices/zephyrr-endobronchial-valve-system-p180002. Accessed 2019 Dec 5.
22. Health Canada. Spiration Valve System (SVS); licence no. 96622. Medical devices active licence listing (MDALL) 2016; https://health-products.canada.ca/mdall-limh/index-eng.jsp. Accessed 2019 Dec 5.
23. U.S. Food and Drug Administration. Listing of CDRH humanitarian device exemptions. 2019; https://www.fda.gov/medical-devices/hde-approvals/listing-cdrh-humanitarian-device-exemptions. Accessed 2020 Jan 10.
24. Hartman JE, Klooster K, Groen H, ten Hacken NHT, Slebos DJ. Cost-effectiveness of endobronchial valve treatment in patients with severe emphysema compared to standard medical care. Respirology. 2018;23(9):835-841.
25. Pietzsch JB, Garner A, Herth FJ. Cost-effectiveness of endobronchial valve therapy for severe emphysema: a model-based projection based on the VENT study. Respiration. 2014;88(5):389-398.
26. Public Health Agency of Canada. Chronic obstructive pulmonary disease (COPD) in Canada. Data Blog 2018; https://health-infobase.canada.ca/datalab/copd-blog.html. Accessed 2020 Jan 29.
27. Report from the Canadian Chronic Disease Surveillance System: Asthma and chronic obstructive pulmonary disease (COPD) in Canada, 2018. Ottawa (ON): Public Health Agency of Canada; 2018: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/asthma-chronic-obstructive-pulmonary-disease-canada-2018.html. Accessed 2020 Jan 29.
28. Han MK, Dransfield M, Martinez FJ. Chronic obstructive pulmonary disease: Definition, clinical manifestations, diagnosis, and staging. In: Post TW, ed. UpToDate. Waltham (MA): UpToDate; 2019: https://www.uptodate.com/. Accessed 2019 Oct 21.
29. Akuthota P, Litmanovich D, Zutler M, et al. An evidence-based estimate on the size of the potential patient pool for lung volume reduction surgery. Ann Thorac Surg. 2012;94(1):205-211.
30. Mansfield C, Sutphin J, Shriner K, Criner GJ, Celli BR. Patient preferences for endobronchial valve treatment of severe emphysema. Chronic Obstr Pulm Dis. 2019;6(1):51-63.
31. Pietzsch JB, Busca R, Rott C, et al. Adoption patterns of bronchoscopic lung volume reduction procedures in Germany and predicted procedure volumes for other European countries. Respiration. 2019;97(1):34-41.
32. Hopkinson NS, Molyneux A, Pink J, Harrisingh MC. Chronic obstructive pulmonary disease: diagnosis and management: summary of updated NICE guidance. BMJ. 2019;366:l4486.
33. Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: A report from the Canadian Thoracic Society COPD Clinical Assembly. Can Respir J. 2015;22(3):147-152.
34. Tran K, Ford C. Endobronchial valves for the management of emphysema: a review of clinical effectiveness. CADTH Rapid response report: summary with critical appraisal. Ottawa (ON): CADTH; 2019: https://cadth.ca/endobronchial-valves-management-emphysema-review-clinical-effectiveness. Accessed 2019 Dec 5.
35. Li S, Wang G, Wang C, et al. The REACH Trial: a randomized controlled trial assessing the safety and effectiveness of the Spiration(R) Valve System in the treatment of severe emphysema. Respiration. 2019;97(5):416-427.
36. Majid A, Labarca G, Uribe JP, et al. Efficacy of the Spiration Valve System in patients with severe heterogeneous emphysema: a systematic review and meta-analysis. Respiration. 2020;99(1):62-72.
37. Gompelmann D, Heinhold T, Rotting M, et al. Long-term follow up after endoscopic valve therapy in patients with severe emphysema. Ther Adv Respir Dis. 2019;13:1753466619866101.
38. U.S. Food and Drug Administration. MAUDE - Manufacturer and User Facility Device Experience. 2020; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM. Accessed 2020 Jan 24.
39. Hartman JE, Vanfleteren L, van Rikxoort EM, Klooster K, Slebos DJ. Endobronchial valves for severe emphysema. Eur Respir Rev. 2019;28(152).
40. Labarca G, Uribe JP, Pacheco C, et al. Bronchoscopic lung volume reduction with endobronchial Zephyr valves for severe emphysema: A systematic review and meta-analysis. Respiration. 2019;98(3):268-278.
41. Gompelmann D, Gerovasili V, Kontogianni K, et al. Endoscopic valve removal >180 days since implantation in patients with severe emphysema. Respiration. 2018;96(4):348-354.
42. Klooster K, Hartman JE, Ten Hacken NH, Slebos DJ. One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration. 2017;93(2):112-121.
43. Sarmand N, Gompelmann D, Kontogianni K, Polke M, Herth FJ, Eberhardt R. New bacterial growth in bronchial secretions after bronchoscopic valve implantation. Int J Chron Obstruct Pulmon Dis. 2018;13:565-570.
44. U.S. Food and Drug Administration. Technical considerations for non-clinical assessment of medical devices containing nitinol - draft guidance for industry and Food and Drug Administration staff. 2019; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-non-clinical-assessment-medical-devices-containing-nitinol. Accessed 2019 Nov 22.
45. Biological responses to metal implants. Rockville (MD): U.S. Food and Drug Administration; 2019: https://www.fda.gov/media/132446/download. Accessed 2019 Nov 14.
46. Franzen DP, Lang C, Agorastos N, Freitag L, Kohler M, Schmid-Grendelmeier P. Evaluation of nickel release from endobronchial valves as a possible cause of hypersensitivity pneumonitis in a patient treated with bronchoscopic lung volume reduction. Int Arch Allergy Immunol. 2017;174(3-4):144-150.
47. National Institute for Health and Care Excellence. Bronchoscopic thermal vapour ablation for upper-lobe emphysema. (Interventional Procedures Guidance IPG652) 2019; https://www.nice.org.uk/guidance/ipg652. Accessed 2020 Jan 24.
48. Polke M, Rotting M, Sarmand N, et al. Interventional therapy in patients with severe emphysema: evaluation of contraindications and their incidence. Ther Adv Respir Dis. 2019;13:1753466619835494.
49. Slebos DJ, Shah PL, Herth FJF, et al. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe chronic obstructive pulmonary disease (AIRFLOW). A multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(12):1477-1486.
50. Right Air. Future of ventilation: help COPD patients breathe easy again. 2020; https://www.rightair.io/. Accessed 2020 Jan 22.
51. Ewing R. How a wear-anywhere vest for COPD evolved. Penn Today. 2019. https://penntoday.upenn.edu/news/how-wear-anywhere-vest-copd-evolved. Accessed 2020 Jan 22.
52. Buttery S, Kemp SV, Shah PL, et al. CELEB trial: Comparative Effectiveness of Lung volume reduction surgery for Emphysema and Bronchoscopic lung volume reduction with valve placement: a protocol for a randomised controlled trial. BMJ Open. 2018;8(10):e021368.
53. UK lung volume reduction trial (ISRCTN16371361). ISRCTN Registry. London (GB): BioMed Central / BMC (Springer Nature); 2017: http://www.isrctn.com/ISRCTN16371361. Accessed 2020 Jan 28.
54. Slebos DJ. Mind the Gap (Trial NL4905 / NTR5007). Utrecht (NL): Netherlands Trial Register; 2014: https://www.trialregister.nl/trial/4905. Accessed 2020 Jan 28.
55. Hartman JE, Klooster K, Slebos D-J. From bench to bedside: implementation of endobronchial valve treatment for patients with advanced emphysema in routine clinical care. Respiration. 2020;99(2):187-188.
56. Criner GJ, Sue R, Wright S, et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med. 2018;198(9):1151-1164.
57. Darwiche K, Aigner C. Clinical management of lung volume reduction in end stage emphysema patients. J Thorac Dis. 2018;10(Suppl 23):S2732-s2737.
58. Thiruvenkatarajan V, Maycock T, Grosser D, Currie J. Anaesthetic management for endobronchial valve insertion: lessons learned from a single centre retrospective series and a literature review. BMC Anesthesiol. 2018;18(1):206.
59. van Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019;7(4):313-324.
About this Document
Authors: Leigh-Ann Topfer, Caitlyn Ford, Melissa Walter
Acknowledgement: We would like to thank Khai Tran for his work on the Rapid Response Report. CADTH would like to thank the external peer reviewers for their comments on an earlier draft of this bulletin.
Cite as: Endobronchial Valves for the Treatment of Severe Emphysema. Ottawa: CADTH; 2020 Mar. (CADTH Issues in Emerging Health Technologies; issue 185).
ISSN: 1488-6324
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policymakers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein do not necessarily reflect the views of Health Canada, Canada’s provincial or territorial governments, other CADTH funders, or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
