T2Lyme Panel: A New Lyme Disease Diagnostic Assay
Reported cases of Lyme disease in Canada have increased more than six-fold from 2009 to 2016.1 A significant portion of cases are classified as “probable” rather than “confirmed,”1 highlighting the uncertainty of standard diagnostic tools. A rapid diagnostic assay such as T2Lyme Panel has the potential to provide a confirmed, earlier diagnosis for Canadians with suspected Lyme disease.
Current Practice
Lyme disease in its early stages is clinically manifested by fever, fatigue, myalgia, and the presence of a rash known as erythema migrans.2 Most people develop symptoms within seven, and up to 30, days of the initial tick bite, but some individuals show no signs at all.3 NICE guidelines recommend that physicians provide diagnosis and commence treatment without laboratory confirmation if the hallmark rash is present.4 Oral doxycycline or intravenous ceftriaxone is administered, depending on symptoms.4
Guidelines from the Centers for Disease Control and Prevention recommend a two-tiered serological testing process for Lyme disease: positive enzyme immunoassay followed by western immunoblot or two enzyme immunoassays.5 Issues with serological testing include high variability between labs and low sensitivity in identifying early disease progression.2 Antibodies detected by these assays generally appear two to four weeks after exposure to Lyme disease and peak around six weeks to three months.6 Turnaround times, according to the Public Health Ontario laboratory, are less than seven days for non-reactive results, 14 days for reactive results, and 21 days if samples are being tested for the European variant of Lyme disease.7
How It Works
T2Lyme Panel, developed by T2 Biosystems, is a polymerase chain reaction-based assay that directly detects bacterial Borrelia DNA in whole blood samples from patients with early-stage Lyme disease.2 Unlike traditional polymerase chain reaction, there are no additional steps for DNA extraction or purification.2 Superparamagnetic particles bind to amplified target DNA in the T2Dx Instrument — a benchtop magnetic resonance reader.2 The clustering of particles alters the measured resonance signal, indicating the presence of Borrelia DNA.2 While the turnaround time for T2Lyme is unknown, the mean time to detection for a similar assay using the same magnetic resonance reader is four hours.8
Who Might Benefit?
A DNA-based assay may be useful for diagnosis prior to the onset of clinical symptoms, with a faster turnaround time compared with serological testing.9 Rapid diagnosis is prudent for anyone suspected of having Lyme disease. It can be especially valuable in cases that present symptoms of central nervous system infection, uveitis, or cardiac complications such as complete heart block.4 Furthermore, almost one-fifth of infections acquired in Canada from 2009 to 2015 did not present erythema migrans,1 a hallmark clinical feature of Lyme disease, which emphasizes the need for sensitive molecular assays to confirm diagnosis.
Across Canada, Lyme disease is most prevalent in Nova Scotia, with 34.4 cases per 100,000 people reported in 2016.10 Lyme disease risk areas have increased over time in Nova Scotia, Manitoba, Ontario, Quebec, and New Brunswick.1 Under-detection is a public health concern: comparison of cross-border disease incidence with bordering US states and counties suggests that only 3.6% to 9.8% of Lyme disease cases are diagnosed.11 Assays that are less prone to false-negative results can better inform health care decisions and provide currently undiagnosed Canadians access to necessary treatment.
Availability in Canada
T2Lyme Panel is currently undergoing clinical testing and has so far not been approved for use. T2Dx Instrument, the component that assesses whole blood samples, is FDA-cleared for other applications.12
What Does It Cost?
The cost of T2Lyme Panel is currently unavailable.
What is the Evidence?
The clinical efficacy of T2Lyme Panel compared with biopsy-obtained culture and serological testing in patients suspected of early Lyme disease is being evaluated in an ongoing non-randomized clinical trial.13 The study was scheduled for completion in late October of 2019.13 A small-scale pre-clinical study analyzed samples from patients with both confirmed and suspected Lyme disease, but did not carry out statistical analysis.2 Currently, there is a gap in published evidence to support the effectiveness of this assay.
Related Developments
Similar whole blood assays that also run on the T2Dx Instrument — T2Bacteria Panel for sepsis-causing bacteria and T2Candida Panel for invasive candidiasis — have been cleared by the FDA.12
Other rapid Lyme disease diagnostic tools have recently been introduced to the market. Quidel Corporation’s Sofia 2 Lyme FIA, a fluorescent immunoassay that detects immunoglobulins M and G antibodies, tests serum or plasma and provides results in 10 minutes.14 It was approved for sale in the US in 2018.14 Point-of-Care Nanotrap Lyme Antigen Test System, developed by Ceres Nanosciences, concentrates and detects antigens from a urine sample.15 It was granted the FDA designation of “Breakthrough Device” in 2018.15
Currently in development, mChip-Ld uses microfluidic cassettes and photodiodes to detect Lyme disease antigens.16 In a 2019 pre-clinical study, the point-of-care assay demonstrated greater sensitivity than standard two-tiered serological testing.16
A 2019 systematic review on unconventional diagnostic tests for Lyme disease highlighted various strategies in development, including novel antigen targets for immunoassays and tests exploring cellular immunity.17 The authors recommended further investigation into the efficacy of rapid tests and noted that assays similar to the T2Lyme Panel have lower sensitivities than laboratory-based diagnostic measures.17
Looking Ahead
Historically, clinical guidelines for Lyme disease have been conflicting and controversial. The classification of Lyme disease as a chronic condition, the significance of post-treatment symptoms, and the potential risks and benefits of antibiotic retreatment are topics of ongoing discussion between patients and health professionals.18 Thus, it is prudent to take patient experience into consideration when introducing new diagnostic or treatment interventions for Lyme disease.
Current serological methods indirectly test for the presence of Borrelia burgdorferi and require a window of at least two to three weeks to rule out a false-negative19 — which provides a convincing rationale for the development of rapid diagnosis tests. However, until the sensitivity and specificity of the T2Lyme assay are established, its clinical application in guiding treatment remains unknown.
Author: Diksha Kumar
References
- Gasmi S, Ogden NH, Lindsay LR, et al. Surveillance for Lyme disease in Canada: 2009-2015. Can Commun Dis Rep. 2017;43(10):194-199.
- Snyder JL, Giese H, Bandoski-Gralinski C, et al. T2 magnetic resonance assay-based direct detection of three Lyme disease-related Borrelia species in whole-blood samples. J Clin Microbiol. 2017;55(8):2453-2461.
- Government of Canada. For health professionals: Lyme disease. 2018; https://www.canada.ca/en/public-health/services/diseases/lyme-disease/health-professionals-lyme-disease.html. Accessed 2019 Oct 16.
- National Institute for Health Care and Excellence. Lyme disease. Recommendations. (NICE guideline NG95) 2018; https://www.nice.org.uk/guidance/ng95/chapter/Recommendations. Accessed 2019 Oct 16.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019 Aug;68(32):703.
- Alberta Health Services. Appendix: laboratory testing for Lyme disease in Alberta. Edmonton (AB): Government of Alberta; 2019: https://www.albertahealthservices.ca/assets/wf/plab/wf-provlab-appendix-laboratory-testing-of-lyme-disease-in-alberta.pdf. Accessed 2019 Oct 16.
- Public Health Ontario. Lyme disease – serology. 2017; https://www.publichealthontario.ca/en/laboratory-services/test-information-index/lyme-disease-serology. Accessed 2019 Oct 16.
- Clancy CJ, Nguyen MH. T2 magnetic resonance for the diagnosis of bloodstream infections: charting a path forward. J Antimicrob Chemother. 2018;73(suppl 4):iv2-iv5.
- Snyder J, Bandoski-Gralinski C, Townsend J, et al. Detection of borrelia in early-stage Lyme disease using T2 magnetic resonance [conference abstract]. Open Forum Infect Dis. 2016;3(suppl 1):205.
- Public Health Agency of Canada. Surveillance of Lyme disease. 2018; https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html. Accessed 2019 Oct 16.
- Lloyd VK, Hawkins RG. Under-detection of Lyme disease in Canada. Healthcare (Basel). 2018;6(4):pii: E125.
- T2 Biosystems. T2Lyme Panel. 2019; https://www.t2biosystems.com/products-technology-ous/t2lyme-2/. Accessed 2019 Oct 16.
- T2 Biosystems. NCT03581279: Detection of Borrelia bacteria in early stage Lyme borreliosis using the T2Lyme panel. Bethesda (MD): U.S. National Library of Medicine; 2019: https://clinicaltrials.gov/ct2/show/record/NCT03581279. Accessed 2019 Oct 16.
- U.S. Food and Drug Administration. 510(k) substantial equivalence determination decision summary. Silver Spring (MD): FDA; 2018: https://www.accessdata.fda.gov/cdrh_docs/reviews/K173691.pdf. Accessed 2019 Oct 28.
- Ceres Nanosciences. Ceres Nanosciences’ point-of-care Nanotrap® Lyme Antigen Test System granted breakthrough device designation by U.S. Food and Drug Administration. 2018 Jul 12: https://www.ceresnano.com/press-release-breakthrough. Accessed 2019 Oct 28.
- Arumugam S, Nayak S, Williams T, et al. A multiplexed serologic test for diagnosis of Lyme disease for point-of-care use. J Clin Microbiol. 2019 Oct 9: pii: JCM.01142-01119. [Epub ahead of print].
- Raffetin A, Saunier A, Bouiller K, et al. Unconventional diagnostic tests for Lyme borreliosis: a systematic review. Clin Microbiol Infect. 2019;12:12.
- Maloney EL. Controversies in persistent (chronic) Lyme disease. J Infus Nurs. 2016;39(6):369-375.
- Schutzer SE, Body BA, Boyle J, et al. Direct diagnostic tests for Lyme disease. Clin Infect Dis. 2019;68(6):1052-1057.
New Surgical Robot Looks to Transform Minimal Access Surgery
CMR Surgical has created a new surgical robotic system to meet the complex needs of minimal access surgery. The device was designed to easily integrate into operating rooms, reduce surgeon fatigue, and enhance access to minimally invasive surgery.
How It Works
The Versius robotic system is a small, multi-port, modular unit designed for a range of minimal access, or laparoscopic, procedures.1 It is composed of an operator console and individually cart-mounted arms that can be positioned around the operating table. The system allows the surgeon to either sit or stand while operating the controls. The surgeon controls the arms from within the operating room with hand-held joystick controllers and a 3-D screen. The robotic arms have similar dexterity and range of motion as human arms and wrists,1 and reportedly reduces the amount of time required to learn to operate the Versius system.2 Versius is said to be quick to set up and take down. It is portable between operating rooms, which could allow for the system to be used more often compared to existing robots that are confined to one dedicated operating room.2
A preclinical assessment of the Versius system used a cadaver model to demonstrate the theoretical advantage of two surgical teams being able to work simultaneously in two surgical fields.3 For complex cases where two surgical fields are required, the Versius system may add value by allowing two surgical teams to operate at once on the same patient, thus reducing the total operative time and total anesthesia time. Operating in two surgical fields at once is not always possible with conventional laparoscopy or open surgery because of space constraints.3
Who Might Benefit?
The Versius system is intended to be used across a range of minimal access surgeries1 and has the potential to benefit a large number of patients. It is unknown how many minimal access surgeries are performed in Canada each year.
Availability in Canada
Versius is not currently licensed for use by Health Canada.4 CMR Surgical has reported that the Versius system was approved for use in Europe in March of 2019.5 No similar modular robotic surgery devices were identified that are currently available in Canada.
What Does It Cost?
The cost of the Versius system is unavailable. CMR Surgical has indicated that it would like to bring the cost of robot-assisted surgery closer to that of conventional laparoscopic surgery6 (Ashley Davis-Marin, Senior Communications Executive, CMR Surgical, Cambridge, UK: personal communication, 2019 Aug 8). CMR Surgical has proposed using a service contract (i.e., an annual fee and a commitment for a number of years in exchange for no-charge disposables and instruments) rather than a standard pricing model (e.g., consumers purchase the machine and disposables).7
Current Practice
Current approaches for minimal access surgery include conventional (i.e., not robot-assisted) laparoscopy and robotic laparoscopy (e.g., da Vinci Surgical Systems). Conventional laparoscopy can strain the surgeons physically and mentally, and a surgeon’s hand tremors can cause instability of the surgical instruments.8 The da Vinci Surgical Systems, which have to be installed in a dedicated operating room, has substantial cost and infrastructure requirements9 and does not provide sensory feedback.8 A 2017 Health Quality Ontario health technology assessment of robot-assisted radical prostatectomy found that the costs of using the da Vinci Systems were relatively high, whereas the health benefits were relatively small.10
What is the Evidence?
The first clinical trial of Versius is currently being conducted in India.11 This clinical trial aims to recruit 270 patients who will undergo minimal access gynecological and other forms of surgery using the Versius system, and the trial will evaluate the safety and efficacy of the Versius surgical robotic system.11 In May 2019, CMR Surgical announced that Versius had successfully been used to complete 30 laparoscopic procedures as part of that clinical trial and that no adverse events were reported in a 30-day follow-up period.5 No other clinical trial evidence is available.
Safety
A patient safety concern with robot-assisted surgical devices in general is the possibility of the device malfunctioning during surgery.9
Issues to Consider
An important consideration for the Versius system will be training the surgical teams to use the system. Surgeons will have to be trained in the technical aspects of the Versius system and also trained to perform specific surgical procedures using the system.9 CMR Surgical offers training for the Versius system that includes online modules, a virtual trainer, a residential training course, and ongoing product support. Other issues to consider with robot-assisted surgery systems in general are the additional time and resources needed to prepare, clean, and maintain the system.9
Looking Ahead
An observational, multi-centred database has also been established in the UK to record safety information from patients who have had surgery using the Versius system.12 The registry is designed to collect information about the surgery (e.g., how long the operation took to complete), as well as any complications during or after the surgery.12 Results from the ongoing clinical trial and registry will help to inform the best use of this technology in the future. However, there is no indication when this technology might be available for use in Canada.
Author: Kendra Brett
References
- CMR Surgical. Versius surgical robotic system. 2019: https://cmrsurgical.com/versius/. Accessed 2019 Aug 12.
- Walsh F. New Versius robot surgery system coming to NHS. BBC News. 2018: https://www.bbc.com/news/health-45370642. Accessed 2019 Aug 12.
- Atallah S, Parra-Davila E, Melani AGF. Assessment of the Versius surgical robotic system for dual-field synchronous transanal total mesorectal excision (taTME) in a preclinical model: will tomorrow's surgical robots promise newfound options? Tech Coloproctol. 2019;23(5):471-477.
- Health Canada. Medical Devices Active Licence Listing (MDALL). 2019: https://health-products.canada.ca/mdall-limh/index-eng.jsp. Accessed 2019 Aug 12.
- CMR Surgical. CMR Surgical successfully completes first set of robotically assisted surgical procedures in humans. 2019: https://cmrsurgical.com/cmr-surgical-successfully-completes-first-set-of-robotically-assisted-surgical-procedures-in-humans/. Accessed 2019 Aug 8.
- Cairns E. Vantage point – The new generation of surgical robots aims for economy. Vantage. 2015: https://www.evaluate.com/vantage/articles/analysis/vantage-point-new-generation-surgical-robots-aims-economy. Accessed 2019 Aug 12.
- Cairns E. Interview – CMR Surgical has $100m and a plan to keep busy. Vantage. 2018: https://www.evaluate.com/vantage/articles/interviews/interview-cmr-surgical-has-100m-and-plan-keep-busy. Accessed 2019 Aug 12.
- Peters BS, Armijo PR, Krause C, Choudhury SA, Oleynikov D. Review of emerging surgical robotic technology. Surg Endosc. 2018;32(4):1636-1655.
- Emerging robotic-assisted surgery systems. Plymouth Meeting (PA): ECRI Institute; 2017: www.ecri.org. Accessed 2019 Aug 12.
- Health Quality Ontario. Robotic surgical system for radical prostatectomy: a health technology assessment. Ontario Health Technology Assessment Series. 2017;17(11):1-172.
- CMR Surgical. A prospective clinical study to evaluate the safety and performance of the Versius surgical robotic system. International Clinical Trials Registry Platform. International Clinical Trials Registry Platform. Geneva (CH): World Health Organization; 2019: http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/02/017872. Accessed 2019 Aug 12.
- CMR Surgical. A database to record safety information from patients who have had surgery using the Versius Surgical Robotic System. International Clincal Trials Registry Platform. Geneva (CH): World Health Organization; 2019:
Auricular Neurostimulation for Opioid Withdrawal
In the first nine months of 2018, more than 3,200 Canadians died from suspected opioid overdoses.1 Initially designed to treat pain, wearable auricular peripheral nerve field stimulation devices such as NSS-2 BRIDGE (Innovative Health Solutions, Inc) are being investigated as an option to alleviate opioid withdrawal symptoms that may encourage people to seek treatment.2
How It Works
The treatment of opioid use disorder consists of short-term withdrawal management and long-term medication-assisted therapy.3 Before transitioning into long-term opioid agonist treatment (e.g., methadone, buprenorphine/naloxone) or opioid antagonist treatment (e.g., naltrexone), immediate withdrawal symptoms such as nausea, insomnia, sweating, increased heart rate, and anxiety need to be addressed.4
Neurostimulation devices such as the NSS-BRIDGE have been shown to help alleviate some withdrawal symptoms2 by stimulating nerve fields that dampen signalling to the amygdala that is associated with fear conditioning, pain processing, and the emotional state of opioid withdrawal.5-7 The amygdala is modulated by the brain stem, which has cranial nerves that project to branches in the external ear forming auricular nerve fields.2,8 As neurostimulation of auricular nerve fields dampens signalling by the amygdala, the severity of withdrawal symptoms may be reduced.2
These wearable devices consist mainly of two parts: a generator and multiple leads. With a battery lasting five days, the BRIDGE generator is attached, by an adhesive, behind the ear.2 The four leads on the BRIDGE generator contain 2 mm titanium needles, which are inserted around the ear to stimulate branches of cranial and occipital nerves.2 Transillumination with a bright light helps guide the placement of needles.2 The BRIDGE device is designed to be left on for five consecutive days and help individuals transition into long-term, medication-assisted therapy.2
The placement of NSS-2 BRIDGE requires minimal training (i.e., an online module and site visit), and is performed in outpatient clinics by trained physicians and physician extenders.2
Who Might Benefit?
As opioid-related fatalities continue to rise in Canada, opioid overdoses have surpassed car accidents as cause of death;9 between 2016 and September 2018, at least 10,300 Canadians died due to suspected opioid-related overdose.1 In 2018, one in 10 people who used opioid pain medications reported problematic use, which includes taking higher quantities than directed, tampering with a drug before using it, and taking it for non‒pain-related reasons.10 Opioid prescriptions lasting as short as five days may increase the chances of long-term use.11 Furthermore, it is estimated that 94% of deaths caused by opioid overdoses are accidents.12 By reducing the fear of experiencing opioid withdrawal symptoms, auricular neurostimulation devices may help promote successful induction, allowing individuals who are seeking treatment to safely start medication-assisted therapy.2
Availability in Canada
NSS-2 BRIDGE is not currently available. Innovative Health Solutions Inc plans to apply for regulatory approval in Canada in 2020. (Dr. Tom Carrico, Chief Regulatory Officer, Innovative Health Solutions, Inc., Versailles, IN: personal communication, 2019 July 30).
What Does It Cost?
Canadian costs were not available. In the US, the current list price of NSS-2 BRIDGE is US$1,195.
Current Practice
As opioid use disorder is a chronic relapsing condition with high mortality rates, most people seeking treatment receive pharmacotherapy and/or psychosocial interventions.3 Similar to commonly prescribed opioids for pain, the medications used to treat opioid use disorder also attach to opioid receptors in the brain.13
The current Canadian guideline lists buprenorphine/naloxone as the preferred first-line treatment, as it has been found to have a better safety profile, fewer drug interactions, and allows for take-home dosing when compared with methadone.3,11,14 However, daily witnessed ingestion of methadone can also be used as a first-line treatment when buprenorphine/naloxone is contraindicated, or when close follow-up is required for those who inject heroin and/or for those with social instability.3
When first-line treatment is ineffective or contraindicated, a second-line treatment option is slow-release oral morphine.3,11,14 Because of limited benefits, loss of tolerance to opioids, and high relapse rates, naltrexone is usually only considered when opioid agonist treatment is contraindicated.3 The Canadian guideline recommends against using withdrawal management alone without the use of long-term medication-assisted therapy.3 Additionally, psychosocial supports (e.g., employment, social assistance) can be used in conjunction with pharmacotherapy.3
Published Studies and Resources
One uncontrolled, open-label retrospective study of the NSS-2 BRIDGE device included 73 participants who were undergoing supervised withdrawal therapy for opioid use disorder.2 This study investigated the effect of the BRIDGE device on clinical opioid withdrawal scale scores and measured the proportion of participants who transitioned to naltrexone detoxification therapy after five days of using the device.2 Mean clinical opioid withdrawal scale scores were reduced from 20.1 to 7.5 (a 62.7% reduction) and 3.1 (a 84.6% reduction) after 20 and 60 minutes of neurostimulation, respectively.2 Overall, 64 of the participants (88%) successfully transitioned to medication-assisted therapy.2
One poster presentation (2019) reported outcomes data of the first 18 months of a prospective study in an obstetric opioid use disorders clinic.15 Out of 367 participants, 14 participants used the BRIDGE device in addition to buprenorphine or methadone and intensive behavioural therapy.15 Of the 14 participants, 10 successfully transitioned to naltrexone detoxification therapy. This study also collected data on neonatal abstinence syndrome rates, relapse rates at delivery, and pregnancy outcomes.15
Safety
Neurostimulation devices tend to be well-tolerated because of the flexibility to stop treatment at any time.16 No side effects were identified in the 73 participants using the BRIDGE device for opioid withdrawal.2 In a retrospective cohort study with more than 1,200 individuals using auricular neurostimulation devices for various indications, minimal to no side effects were identified.17 In 19,312 needle placements, there were 11 incidences of localized bleeding (0.057%) and 11 incidences of localized dermatitis (0.062%).17
Issues to Consider
As opioid use disorder disproportionately affects individuals with lower socioeconomic status,18 funding may be a barrier to access these devices. Auricular neurostimulation devices must be applied by a trained provider.2 Additionally, the use of the BRIDGE device is contraindicated in individuals with conditions such as hemophilia, cardiac pacemakers, or psoriasis vulgaris.19
Related Developments
Innovative Health Solutions, Inc has recently obtained clearance from the US FDA for IB-Stim — a non-surgical device that aims to treat functional abdominal pain related to irritable bowel syndrome in adolescent patients.20 In a randomized, sham-controlled trial involving 115 subjects, the neurostimulation group exhibited a greater decrease in pain frequency-severity-duration scores than those in the sham group.21
Drug Relief, a similar auricular neurostimulation device made by DyAnsys Inc., has also been cleared by the FDA to treat opioid withdrawal symptoms.22 Additionally, an ongoing randomized controlled trial is studying the effectiveness of the Primary Relief (DyAnsys) auricular neurostimulation device for post-caesarean pain.23
From a patient follow-up perspective, wearable wrist biosensors have been developed to monitor physiological changes (i.e., electrodermal activity, skin temperature, whole-body acceleration) before, during, and after opioid use.24 Despite the potential benefits of continuous and remote monitoring, patient uptake, and adherence may be issues that require further investigation.24
Currently unavailable in Canada, lofexidine hydrochloride (Lucemyra) was recently approved by the US FDA as the first non-opioid treatment for opioid withdrawal symptoms.25 Lucemyra does not reduce psychological cravings, but rather alleviates symptoms such as sweating, nausea, and rapid heart rate.25 Researchers at The Scripps Research Institute in California have conducted preclinical studies of a novel opioid vaccine.26 The vaccine is designed to teach the immune system to identify and block opioid drugs from entering the brain, with the goal of preventing overdoses and relapses.26
Looking Ahead
Two ongoing clinical trials are investigating the use of the BRIDGE device in opioid withdrawal therapy.27 28 One of the two studies is a randomized controlled trial comparing the device to the standard of care treatment (i.e., methadone wean) in preventing withdrawal symptoms among pediatric patients in the intensive care unit.28 Further studies are required to fully elucidate the mechanism of action of auricular neurostimulation.2
Author: Yan Li
References
- Public Health Agency of Canada. Updated numbers on opioid-related overdose deaths in Canada. 2019; https://www.canada.ca/en/public-health/news/2019/04/updated-numbers-on-opioid-related-overdose-deaths-in-canada.html. Accessed 2019 Sep 4.
- Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am J Drug Alcohol Abuse. 2018;44(1):56-63.
- CRISM national guideline for the clinical management of opioid use disorder. Canadian Research Initiative in Substance Misuse; 2018: https://crism.ca/wp-content/uploads/2018/03/CRISM_NationalGuideline_OUD-ENG.pdf. Accessed 2019 Aug 12.
- Volkow ND, McLellan AT. Opioid abuse in chronic pain--misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263.
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221-234.
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642-645.
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217-238.
- Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8(3):624-636.
- Centre for Addiction and Mental Health. Tackling Canada’s opioid crisis: help for patients and care providers. 2018; https://www.camh.ca/en/camh-news-and-stories/tackling-canadas-opioid-crisis-help-for-patients-and-care-providers. Accessed 2019 Sep 24.
- StatsCan. Canadian Community Health Survey, 2018. The Daily 2019; https://www150.statcan.gc.ca/n1/daily-quotidien/190625/dq190625b-eng.htm. Accessed 2019 Sep 24.
- Korownyk C, Perry D, Ton J, et al. Managing opioid use disorder in primary care: PEER simplified guideline. Can Fam Physician. 2019;65(5):321-330.
- Health Canada. Canada’s opioid crisis (fact sheet). 2019; https://www.canada.ca/en/health-canada/services/publications/healthy-living/canada-opioid-crisis-fact-sheet.html. Accessed 2019 Sep 24.
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1(1):13-20.
- Bruneau J, Ahamad K, Goyer ME, et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ. 2018;190(9):E247-e257.
- Towers CV, Katz E, Visconti K, Howard B, Wolfe L, Fortner K. 142: Opioid use disorder (OUD) clinic - first 18 months; detoxification and Bridge Device. Am J Obstet Gynecol. 2019;220(1 Supplement):S109-S110.
- Mercante B, Deriu F, Rangon CM. Auricular neuromodulation: the emerging concept beyond the stimulation of vagus and trigeminal nerves. Medicines (Basel). 2018;5(1).
- Roberts A, Sithole A, Sedghi M, Walker CA, Quinn TM. Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study. Med Devices (Auckl). 2016;9:389-393.
- Ghertner R, Groves L. The opioid crisis and economic opportunity: geographic and economic trends. Washington (DC): Office of The Assistant Secretary for Planning and Evaluation; 2018: https://aspe.hhs.gov/system/files/pdf/259261/ASPEEconomicOpportunityOpioidCrisis.pdf. Accessed 2019 Aug 12.
- U.S. Food and Drug Administration. FDA grants marketing authorization of the first device for use in helping to reduce the symptoms of opioid withdrawal. 2017; https://www.fda.gov/news-events/press-announcements/fda-grants-marketing-authorization-first-device-use-helping-reduce-symptoms-opioid-withdrawal. Accessed 2019 Aug 12.
- U.S. Food and Drug Administration. Clearance letter to Innovative Health Solutions. 2019; https://www.accessdata.fda.gov/cdrh_docs/pdf18/DEN180057.pdf. Accessed 2019 Oct 22.
- Kovacic K, Hainsworth K, Sood M, et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol. 2017;2(10):727-737.
- DyAnsys. Drug Relief®. 2019; https://www.dyansys.com/products-applications/products/drug-relief. Accessed 2019 Aug 12.
- DyAnsys Inc. NCT03829774: To study and evaluate the effectiveness of treatment by percutaneous electrical neurostimulation (PENS) for post-operative pain in Cesarean section patients using Primary Relief v 2.0 (POPS). Clinicaltrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019: https://clinicaltrials.gov/ct2/show/NCT03829774?term=Percutaneous+Electrical+Nerve+Field+Stimulation&rank=6. Accessed 2019 Oct 29.
- Nuamah JK, Sasangohar F, Erraguntla M, Mehta RK. The past, present and future of opioid withdrawal assessment: a scoping review of scales and technologies. BMC Med Inform Decis Mak. 2019;19(1):113.
- Myers-O'Shea B. How Lucemrya reduces opioid withdrawal symptoms. PharmacyTimes 2018; https://www.pharmacytimes.com/contributor/brittany-myers-pharmd/2018/07/how-lucemrya-reduces-opioid-withdrawal-symptoms. Accessed 2019 Sep 27.
- Bates M. Tackling an epidemic: new and emerging opioid addiction treatments offer hope for solutions to this crisis. IEEE Pulse. 2018;9(2):22-25.
- The Cleveland Clinic. NCT03762798: Can the Bridge transition opiate use disorder patients in stable recovery from buprenorphine to naltrexone. Clinicaltrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018: https://clinicaltrials.gov/ct2/show/NCT03762798?term=nss-2+bridge&rank=5. Accessed 2019 Oct 29.
- Medical College of Wisconsin. NCT03975192: Neurostimulation for opiate withdrawal in the PICU (NOW). Clinicaltrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019: https://clinicaltrials.gov/ct2/show/NCT03975192?term=Percutaneous+Electrical+Nerve+Field+Stimulation&rank=2. Accessed 2019 Aug 12.
reSET-O Offers a New Option for People With Opioid Use Disorder
The reSET-O prescription digital therapeutic is a new form of treatment support for people with opioid use disorder that can be accessed via a smartphone.
How It Works
reSET-O is a prescription digital therapeutic (PDT) for adults with opioid use disorder (OUD).1 It is a smartphone or tablet application (app) that is used for 84 days to provide cognitive behavioural therapy (CBT) to patients with OUD, while they are also receiving buprenorphine treatment under the supervision of a physician or therapist.1 Patients work their way through the program on the app by following prompts to complete quizzes, and to report medication and substance use, cravings, and triggers.1
In addition to the patient-facing interface, reSET-O also includes a dashboard that health care providers can use to monitor people’s progress through the program.1 Providers can access information on the amount of the program the person has completed, their reports of any use or cravings, as well as any in-clinic drug screening test results.1 reSET-O is available through the major app stores in the US; however, its content is not accessible without a prescription and access code obtained through a physician.1 The manufacturer has stated: “The goal of these apps is to ‘reprogram’ the brain's rewards system once it has been distorted by addictive substances.”2 Currently, reSET-O is indicated for people whose primary language is English and who are comfortable and familiar with the use of apps.1
Who Might Benefit?
Opioid use has become a major public health issue in Canada and has been cited as the main reason why, for the first time, Canadian life expectancy did not increase between 2016 and 2017.3 In 2018, 3.7 million Canadians — or 13% of the population over the age of 14 years — reported using an opioid medication.3 Of those 3.7 million people, close to 600,000 reported using opioids daily or almost daily and about 351,000 (10%) of individuals who had reported using an opioid classified their use as problematic.3 In Canada in 2016, the rate of deaths related to opioids was 7.9 per 100,000 people — or 2,861 deaths.4 In 2017, there were close to 4,000 opioid-related deaths,5 or about 11 deaths per day.6 It is estimated that 94% of opioid-related deaths happen by accident.6
reSET-O is intended to help adults with OUD to stop using opioids and may be an option for people with OUD who are not able to regularly access face-to-face time with a physician or therapist.1
Availability in Canada
reSET-O is not currently available in Canada.7 The manufacturer has indicated it is exploring potential expansion and access in Canada (Dr. Yuri Maricich, Chief Medical Officer, Pear Therapeutics, Boston, MA: personal communication, 2019 Aug 3). The app is available in the US and received FDA approval in December 2018.8
What Does It Cost?
The reSET-O PDT is available for free from the app stores in the US. There is currently no indication of the costs associated with using the app’s content. The app is to be used in conjunction with buprenorphine treatment.
Current Practice
Canadian guidelines recommend that the management of OUD be performed as part of primary health care.5 The guidelines indicate that clinicians should discuss the treatment of OUD with their patients; opioid agonist treatment with buprenorphine/naloxone is recommended as first-line treatment, when feasible.5,9,10 When buprenorphine/naloxone is not working or is not indicated, the guideline recommendations suggest methadone may be considered, instead.9,10 Withdrawal management alone is not recommended because of the increased risk of syringe sharing and death from potential overdose.10 The recommendations indicate that the addition of counselling or psychosocial treatment interventions should be offered to those with OUD5,9 but should not be a mandatory requirement for accessing treatment.9
What Is the Evidence?
The FDA notification states that it reviewed “data from a multi-site, unblinded, controlled 12-week clinical trial of 170 patients who received supervised buprenorphine treatment paired with a behavior therapy program, either with or without the addition of a desktop-based version of reSET-O, which was accessed at the clinic.” However, there is no citation provided and the study could not be identified.8 Studies were identified that evaluated the reSET app for substance use disorder but none for the reSET-O app specifically for OUD.
It should be noted that the following disclaimer is provided on the manufacturer’s website: “reSET-O has not been shown to decrease illicit drug use or improve abstinence in patients with OUD.”1
One ongoing clinical trial of the reSET-O app was identified that is estimated to be completed in December 2019.11
Safety
No specific evidence relating to the safety of the reSET-O app was identified, nor were any safety issues mentioned in the literature that was reviewed.
Related Developments
There are a variety of drug-related mobile health apps available on the market that are used to share drug-related information and advice with users, provide support and interventions for people who use drugs (like reSET), and apps intended for use by health professionals.12
An alternative version of the PDT, reSET, is available for adults with substance use disorder that does not include opioids as the primary substance of misuse.1
Looking Ahead
Research has shown that improvements in health outcomes can be greater when people have more access to their own health information and are engaged with their health.13 While not everyone is immediately comfortable with the addition of personal technologies to health care, their use will likely become a more standard and accepted component in how people’s health is managed as more health-related apps enter the space.
Michelle Clark
References
- reSET® & reSET-O®. Boston (MA): Pear Therapeutics; 2019: https://peartherapeutics.com/products/reset-reset-o/. Accessed 2019 Aug 2.
- Take this app: tech firms tackle opioid crisis with software. The Star Online, 2019 May 22. Pengerang (MY): The Star Online; 2019: https://www.thestar.com.my/tech/tech-news/2019/05/22/take-this-app-tech-firms-tackle-opioid-crisis-with-software/#IZzkfzyo0CZomSvy.99. Accessed 2019 Aug 2.
- Canadian Community Health Survey, 2018. Ottawa (ON): Statistics Canada; 2019: https://www150.statcan.gc.ca/n1/daily-quotidien/190625/dq190625b-eng.htm. Accessed 2019 Aug 6.
- Tackling Canada’s opioid crisis: help for patients and care providers. Toronto (ON): Centre for Addiction and Mental Health; 2018: https://www.camh.ca/en/camh-news-and-stories/tackling-canadas-opioid-crisis-help-for-patients-and-care-providers. Accessed 2019 Aug 2.
- Korownyk C, Perry D, Ton J, et al. Managing opioid use disorder in primary care: PEER simplified guideline. Can Fam Physician. 2019;65(5):321-330.
- Canada’s opioid crisis (fact sheet). Ottawa (ON): Government of Canada; 2019: https://www.canada.ca/en/health-canada/services/publications/healthy-living/canada-opioid-crisis-fact-sheet.html. Accessed 2019 Aug 2.
- Medical devices active license search. Ottawa (ON): Government of Canada; 2019: https://health-products.canada.ca/mdall-limh/prepareSearch-preparerRecherche.do;jsessionid=ADFB7EA930F036ED7AF1D39776A79337?type=active. Accessed 2019 Aug 6.
- FDA clears mobile medical app to help those with opioid use disorder stay in recovery programs [press release]. Silver Spring (MD): U.S. Food and Drug Administration (FDA); 2018: https://www.fda.gov/news-events/press-announcements/fda-clears-mobile-medical-app-help-those-opioid-use-disorder-stay-recovery-programs. Accessed 2019 Aug 2.
- CRISM national guideline for the clinical management of opioid use disorder. Toronto (ON): Canadian Research Initiative in Substance Misuse; 2018: https://crism.ca/wp-content/uploads/2018/03/CRISM_NationalGuideline_OUD-ENG.pdf. Accessed 2019 Aug 2.
- Bruneau J, Ahamad K, Goyer ME, et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ. 2018;190(9):E247-E257.
- Milton S. Hershey Medical Center. NCT03826966: Comprehensive CBT (Cognitive Behavioral Therapy) via reSET app. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019: https://clinicaltrials.gov/ct2/show/NCT03826966. Accessed 2019 Aug 2.
- m-Health applications for responding to drug use and associated harms. Lisbon (PT): European Monitoring Centre for Drugs and Drug Addiction 2018: http://www.emcdda.europa.eu/system/files/publications/10244/EMCDDA%20Papers_m-Health%20applications_Final.pdf. Accessed 2019 Aug 2.
- Annual report 2018-2019: A new day in health care is coming. Toronto (ON): Canada Health Infoway; 2019: https://www.infoway-inforoute.ca/en/component/edocman/3726-annual-report-2018-2019/view-document?Itemid=101. Accessed 2019 Sep 12.
New Monitoring Device for Measuring Patients’ Physiological Responses to Pain
Accurately monitoring pain is difficult, as many of the accepted measures for pain — such as the numerical rating scale and the visual analogue scale — are subjective and rely on asking patients to describe their experiences. Therefore, when a patient is sedated, many of these measures are not available.
The PMD-200 measures multiple physiological parameters and translates them into a score that reflects a person’s physiological response to pain. This device can be used to monitor conscious patients, as well as patients who are under general anesthesia, unable to communicate their experiences.
How It Works
The PMD-200 includes a finger probe that receives signals from four different sensors and transmits them to a bedside monitor. The sensors continuously monitor heart rate, heart rate variability, plethysmograph wave amplitude, skin conductance, skin temperature, and skin temperature fluctuations.1 These data are then analyzed to produce a nociception index score from 0 to 100. Nociception is the central nervous system’s response to noxious stimuli that may be tissue damaging.2 Nociception and nociceptive pain will be referred to interchangeably throughout this article and refers to the body’s physiological response to noxious stimuli. Nociception can be experienced even when a patient is under general anesthesia.
The PMD-200 is the first device that includes an objective indicator, the nociception level index (NoL index), which is informed by multiple physiological parameters and machine-learning algorithms. Notable improvements in the latest version of the device are more efficient sensors and an integrated analogue-to-digital converter.1
Who Might Benefit?
In 2016-2017, there were more than 480,000 in-patient surgeries in Canada — which represents a significant population that might benefit from nociception monitoring.3 The PMD-200 has been tested in the operating room to communicate the nociception of sedated patients. The use of the PMD-200 device in this scenario could help prevent over- or under-usage of opioid analgesics during the perioperative period, which can be associated with negative patient outcomes. Overtreatment with opioid analgesics can lead to opioid-induced hyperalgesia, higher opioid tolerance, and immunosuppression;1 whereas inadequate pain suppression can lead to intraoperative pain, which may stress the body and worsen pain after surgery.4
Earlier versions of the PMD-200 device were studied in conscious subjects.2,5 In awake patients, it is possible to monitor nociception in addition to sensations of pain. A potential use of the device in awake patients would be to provide monitoring in individuals who experience chronic pain. The use of the PMD-200 in populations with chronic pain would increase the potential impact of the device, as it is estimated that one in five Canadians experiences chronic pain.6
The PMD-200 might also be of use to patients who are unable to communicate their pain such as children, and individuals with disabilities that affect speech or communication, although there is no evidence for the use of the device in these populations at this time.
Availability in Canada
The PMD-200 was approved for use in Canada as a Class 3 medical device in August 2017.7 It is unknown how widely used it is.
What Does It Cost?
Cost information for the PMD-200 is not publicly available. In the context of pain monitoring during surgery, the PMD-200 would have additional up-front costs in both purchasing the device and training staff for its use. The PMD-200 may have indirect patient savings if post-operative, post-discharge care requirements are reduced because of decreased post-surgical pain and the reduced use of opioid analgesics.1 However, additional research is needed regarding cost savings, as no evidence was identified at this time.
In the context of pain monitoring for individuals with chronic pain, there may be additional costs to the patient — either financial if they had to purchase their own monitor, or lost opportunity cost if they had to check in with a clinician periodically. The potential for cost savings exists in this population if accurate and objective pain monitoring leads to better patient outcomes.
Current Practice
The 2019 Guidelines to the Practice of Anesthesia prepared by the Canadian Anesthesiologists’ Society detail how patients should be monitored whether they are receiving general anesthesia, regional anesthesia, or procedural sedation.8 The guidelines specify that the duties of an anesthesiologist include the administration of an anesthetic agent, as well as the continual monitoring of the anesthetized patient before, during, and after their procedure.8
A sedated patient is closely monitored by their care team for a variety of signs. Clinicians infer the nociceptive level from clinical signs such as hypertension and tachycardia that are linked to the activation of the autonomic nervous system.1 However, according to a recent systematic review, there is not yet a gold standard for measuring nociceptive pain.9
Published Studies
A single, prospective observational study (reported in two publications) of 26 patients undergoing midline laparotomy in which the PMD-200 was used to monitor patients was identified.1,10 In the study, in addition to the PMD-200, the patients’ heart rates and mean arterial pressures were monitored and the bispectral, or BIS, index was used. Authors concluded that the PMD-200 (using the NoL scale) was better able to distinguish between noxious and non-noxious stimuli than the other measures.1,10
In addition to the single-centre study, an interim analysis of an ongoing randomized controlled trial reported that the PMD200 was used to monitor patients’ NoL index intraoperatively in 28 patients receiving phenylephrine boluses.11 This study is an interim analysis; further investigation of the clinical relevance of the results is required.
There are several clinical trials investigating the NoL index and/or an earlier version of the PMD device.2,5,12-17 These studies have found that NoL monitoring is a promising index to assess the level of nociception in conscious and unconscious patients including patients experiencing acute and chronic pain.2,5,12-17
While the clinical benefits of accurate monitoring of nociceptive pain have been hypothesized, they have yet to be demonstrated clearly in a randomized controlled trial. Research that links nociceptive pain monitoring using the PMD-200 to better patient outcomes is needed.
Safety
No safety information for the PMD-200 was identified.
Related Developments
Nociception monitoring is a growing research interest. While the PMD-200 is the only multiparameter device on the market, there are a few devices that look at one or two parameters.
Single-parameter nociception measures include the ANI‒Analgesia Nociception Index (Mdoloris Medical Systems, Loos, France), which measures heart rate variability and was approved by Health Canada in 2017.18 Two additional single-parameter devices that have not received Health Canada approval are a device that measures pupil diameter — the AlgiScan (IDMed, Marseille, France) and a device that measures micro-fluctuations in skin conductance (Med- Storm Innovation, AS, Oslo, Norway).
Two parameter nociception measures include the surgical pleth index, which measures pulse-wave amplitude and heart beat interval, and the qCON 2000 Monitor (Quantium Medical [Fresenius Kabi], Mataro, Spain), which combines electroencephalogram — EEG — and electromyography — EMG.19
Despite the increased interest in this area, the use of these technologies has yet to have been firmly linked to improved patient outcomes.
Looking Ahead
There are several devices on the market that aim to help monitor nociception during surgery. Devices like the PMD-200 provide previously unavailable objective and accurate data regarding a patient’s physiologic response to pain. It is important to note that these devices have not yet been linked to improved patient outcomes. A 2017 meta-analysis found seven randomized controlled trials on nociception monitoring during anesthesia.20 This meta-analysis associated the use of nociception monitoring devices to a significant reduction of movement events, a non-significant trend toward the reduction of intraoperative-administered opioids and emergence time, but was inconclusive with regard to effects on hemodynamic events, post-operative pain, and opioid consumption.20 For nociception monitoring devices to be more widely used, randomized controlled trials testing their clinical benefit in patients under general anesthesia are required.21
Author: Sarah Jones
References
- Renaud-Roy E, Stockle PA, Maximos S, et al. Correlation between incremental remifentanil doses and the Nociception Level (NOL) Index response after intraoperative noxious stimuli. Can J Anaesth. 2019;66(9):1049-1061.
- Martini CH, Boon M, Broens SJ, et al. Ability of the nociception level, a multiparameter composite of autonomic signals, to detect noxious stimuli during propofol-remifentanil anesthesia. Anesthesiology. 2015;123(3):524-534.
- Canadian Institute of Health Information. Inpatient hospitalization, surgery and newborn statistics, 2017–2018. 2019; https://www.cihi.ca/sites/default/files/document/dad-hmdb-childbirth-quick-stats-2017-2018-en.xlsx. Accessed 2019 Sep 13.
- Ledowski T. Monitoring nociception-getting 'there yet' might be easier with a road map. Br J Anaesth. 2017;119(4):716-717.
- Ben-Israel N, Kliger M, Zuckerman G, Katz Y, Edry R. Monitoring the nociception level: a multi-parameter approach. J Clin Monit Comput. 2013;27(6):659-668.
- Chronic pain in Canada: laying a foundation for action. A report by the Canadian pain task force, June 2019. Ottawa (ON): Health Canada; 2019: https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2019.html#a1.4. Accessed 2019 Sep 11.
- PMD-200 pain monitoring system; licence no. 99488. Medical devices active licence listing (MDALL) 2019; https://health-products.canada.ca/mdall-limh/dispatch-repartition.do?type=active. Accessed 2019 Sep 6.
- Dobson G, Chow L, Flexman A, et al. Guidelines to the practice of anesthesia – revised edition 2019. Can J Anesth. 2018(66):75-108.
- Meijer FS, Niesters M, van Velzen M, et al. Does nociception monitor-guided anesthesia affect opioid consumption? A systematic review of randomized controlled trials. J Clin Monit Comput. 2019;20:20.
- Renaud-Roy E, Stockle PA, Verdonck O, Fortier LP, Richebe P. Impact of different remifentanil doses on the Nociception Level Index response to intra-operative noxious stimuli. Can J Anesth. 2017;64(Suppl 1):1-266.
- Raft J, Coulombe MA, Renaud-Roy E, et al. Impact of intravenous phenylephrine bolus administration on the nociceptive level index (NOL). J Clin Monit Comput. 2019.
- Meijer FS, Martini CH, Broens S, et al. Nociception-guided versus standard care during remifentanil-propofol anesthesia: a randomized controlled trial. Anesthesiology. 2019;130(5):745-755.
- Stockle PA, Julien M, Issa R, et al. Validation of the PMD100 and its NOL Index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol. 2018;84(10):1160-1168.
- Gelinas C, Richebe P. Exploring the use of an innovative technology for pain assessment during mediastinal tube removal in cardiac surgery patients in the intensive care unit: the Nociception Level (NOL) Index TM. Intensive Care Med Exp. 2018;6(Suppl 2):40.
- Bollag L, Jelacic S, Delgado Upegui C, Wu C, Richebe P. The Nociception Level Index (NOL) response to intubation and incision in patients undergoing video-assisted thoracoscopic surgery (VATS) with and without thoracic epidural analgesia. A pilot study. F1000Res. 2018;7:875.
- Edry R, Recea V, Dikust Y, Sessler DI. Preliminary intraoperative validation of the Nociception Level Index: a noninvasive nociception monitor. Anesthesiology. 2016;125(1):193-203.
- Treister R, Kliger M, Zuckerman G, Goor Aryeh I, Eisenberg E. Differentiating between heat pain intensities: the combined effect of multiple autonomic parameters. Pain. 2012;153(9):1807-1814.
- ANI Monitor V2, licence no. 99960. Medical devices active licence listing (MDALL) 2017; https://health-products.canada.ca/mdall-limh/dispatch-repartition.do?type=active. Accessed 2019 Oct 23.
- Ledowski T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123(2):e312-e321.
- Gruenewald M, Dempfle A. Analgesia/nociception monitoring for opioid guidance: meta-analysis of randomized clinical trials. Minerva Anestesiol. 2017;83(2):200-213.
- Funcke S, Pinnschmidt HO, Wesseler S, et al. Guiding opioid administration by 3 different analgesia nociception monitoring indices during general anesthesia alters intraoperative sufentanil consumption and stress hormone release: a randomized controlled pilot study. Anesth Analg. 2019;10:10.
SC+: A Portable Hemodialysis System for Integrated Home and In-Centre Treatment
Traditionally done in a health care setting and more recently at home, hemodialysis is a common treatment for end-stage renal disease. SC+ provides a new option for self-administered hemodialysis in clinics and at home.
How It Works
SC+ is a small hemodialysis system intended for both assisted and self-care hemodialysis either in the home or in a clinic.1 SC+ measures 370 mm by 550 mm by 520 mm and weighs 32 kg.2 The device features a large touch screen interface that guides users through treatment. The interface has been validated with patients and health care practitioners via independent human factors testing.3 SC+ provides high-dose, high-volume dialysis treatment equivalent to larger and more complex machines.1 Rather than reusing internal fluidic circuits, as is common in traditional dialysis machines, SC+ uses a disposable cartridge to generate and manage the dialysate fluid, negating the need for pre-mixed dialysate.1 The disposable cartridge is changed after each use, which means the machine does not need to be disinfected each time.1 The SC+ was designed to be small enough to easily fit into the home and straightforward in use to allow patients to easily transition between a clinic and a home hemodialysis set-up.3
Who Might Benefit?
As of 2019, 4 million Canadians — about one person in 10 — have chronic kidney disease (CKD).4 Approximately 49,000 of those with CKD are being treated for kidney failure.4 SC+ is intended for use by people with end-stage renal disease (ESRD).1 Having ESRD means that a person’s kidneys have reached the end of their useful life and can no longer adequately filter blood.5 Fifty-seven per cent of those being treated for ESRD in Canada are on dialysis and three-quarters of those patients receive their dialysis in a health care setting.4
There are two types of dialysis: peritoneal dialysis and hemodialysis.6 Hemodialysis works by running the patient’s blood through a dialyzer alongside dialysate fluid and the waste products are removed from the blood across a thin membrane.6 Peritoneal dialysis also filters the blood; however, the process takes place within the person’s peritoneal cavity, with the peritoneum acting as a natural membrane.7 Hemodialysis is typically done in-centre, and sometimes at home, with standard hemodialysis machines that can be very bulky and take up a lot of space.6
Hemodialysis is typically done about three times a week in a dialysis centre and each session can take between four to five hours to complete. The in-clinic sessions are done under a strict schedule that does not leave a lot of flexibility for the patient.6
Once a person has been stabilized through standard hemodialysis treatment, they may be eligible for self-care home hemodialysis.6 Moving the treatment setting to the home enables people to plan their dialysis treatments around their lives versus planning their day-to-day activities around their dialysis treatments.6 It is estimated that between 30% and 40% of those who undergo hemodialysis could be capable of performing self-care dialysis.1 As SC+ is smaller than most other home hemodialysis options and has been described as being more user-friendly, it may be appealing to those for whom bulkier machines or those with more complex user-interfaces have not been an option.3
Availability in Canada
A search of Medical Devices Active Licence Listing showed no current licences for SC+ in Canada.8 SC+ received regulatory approval in the EU in 20159 and has been piloted with NHS.1 A commercial launch in the UK is planned for 2020 ( Dr. David Bond, Senior Marketing Executive, Quanta Dialysis Technologies, Alcester, UK: personal communication, 2019 Dec 13) and the company plans to file a 510(k) submission with the FDA in early 2020.1 The manufacturer intends to register SC+ with Health Canada after completion of the FDA submission (Dr. David Bond: personal communication, 2019 Dec).
Another small hemodialysis device intended for home use, the NxStage System One, is available for sale in Canada.10
What Does It Cost?
No specific costs related to SC+ were identified, although it is anticipated that the use of the machine could decrease health care costs by moving treatment from staffed dialysis centres to the home.1
According to the results of a 2018 CADTH Optimal Use Report, home-based hemodialysis and non-assisted peritoneal dialysis therapies are less costly than in-centre hemodialysis.11 Home-based dialysis methods may reduce the need for centralized facilities and travel, or relocation, for patients who live outside of urban centres, and may also offer cost savings, compared with in-centre hemodialysis, for patients and the health care system.11
Current Practice
CKD is often caused by another condition, such as diabetes or high blood pressure, and the initial step to treat CKD is to treat or manage the underlying condition.12 When kidney function falls below a certain level, kidney failure (or ESRD) begins and can affect other areas of the body including the heart, bones, or brain.12 Once a person is diagnosed with ESRD, treatment options include dialysis or kidney transplant, depending on the severity of the kidney damage.12 Dialysis may occur in a hospital or dialysis clinic for patients who are quite sick, or potentially at home for patients who are well enough to care for themselves.6 For patients whose physicians have decided they are a fit for self-care dialysis, either home hemodialysis or peritoneal dialysis is recommended, although there is insufficient high-quality evidence to indicate that either method is a better treatment than in-centre hemodialysis.11
What is the Evidence?
One observational study of the human factors testing of SC+ involving UK health care professionals and patients was identified.3 Seventeen health care professionals and 15 dialysis patients and caregivers underwent four-and-one-half to six hours of training and then used SC+.3 The number and severity of errors were recorded and discussed.3
In the identified observational study,3 29 errors were reported in 1,216 interactions with SC+ that presented an opportunity for error. The authors indicated that the observed errors were related to the users’ familiarity with the device and that none of the errors were related to the safety of SC+.3
Other Information
The manufacturer has also published conference abstracts and posters, one of which describes a multi-centre observational study that assessed the safety, efficacy, and usability of SC+ at sites in the UK.13-16 One abstract outlined the previously mentioned usability study.17 The study participants were adults with ESRD who were dependent on hemodialysis. No adverse events were observed and SC+ was used by participants without difficulty.13-17
Safety
Serious adverse events associated with home hemodialysis are relatively uncommon.18 Common concerns with the safety of home hemodialysis are related to the potential for significant blood loss; however, research has shown these events are rare.19 Vascular access complications and infection can also occur.18 Home hemodialysis has been shown to be as safe as in-centre dialysis.18In the clinical pilot observational study with the NHS, reported in a poster presentation, Quanta reported no adverse events.20
Issues to Consider
Home-based hemodialysis is associated with lower patient travel costs, higher home utility costs, and potential benefits in home and workforce productivity for employed patients.11 Studies of other portable hemodialysis units have demonstrated their usefulness in rural hospitals where patients may not have easy access to traditional in-centre hemodialysis.21 A positive result may be feelings of increased self-worth and empowerment because they are able to take control of a part of their own care.3
Barriers to the uptake of home hemodialysis have been identified that could be considered when deciding whether to implement a home-based dialysis system.3 Patients may be hesitant to turn their homes into treatment facilities or to take up space in their homes with medical equipment.3 There may be a fear of making mistakes when they are responsible for their own treatment and comorbidities may limit their ability to manage their own dialysis sessions safely.3
Related Developments
Other systems intended for self-administered home hemodialysis exist22 and one of these systems, NxStage System One, is available for sale and use in Canada.10
Michelle Clark
References
- Mercadal L, Oriane L, Cecile C, et al. MON-111 Effect of the dialysate calcium concentration and of the mineral bone disease therapies on mortality in the REIN registry [abstract]. Kidney Int Rep. 2019;4(7 Suppl):S349-S350. Presented at: World Congress of Nephrology; 2019 Apr; Melbourne, AU.
- SC+ technical specifications (SM-0027-B V 1.5). Alcester (UK): Quanta Dialysis Technologies.
- Harasemiw O, Day C, Milad JE, Grainger J, Ferguson T, Komenda P. Human factors testing of the Quanta SC+ hemodialysis system: an innovative system for home and clinic use. Hemodial Int. 2019;23(3):306-313.
- Nair S, Gautier J. MON-112 Home Haemodialysis (HHD) with low dialysate volume (LDV) - the green benefit [abstract]. Kidney Int Rep. 2019;4(7 Suppl):S350. Presented at: World Congress of Nephrology; 2019 Apr; Melbourne, AU.
- Damery S, Brown C, Sein K, Nicholas J, Baharani J, Combes G. The prevalence of mild-to-moderate distress in patients with end-stage renal disease: results from a patient survey using the emotion thermometers in four hospital trusts in the West Midlands, UK. BMJ Open. 2019;9(5):e027982.
- Shah KK, Murtagh FEM, McGeechan K, et al. Health-related quality of life and well-being in people over 75 years of age with end-stage kidney disease managed with dialysis or comprehensive conservative care: a cross-sectional study in the UK and Australia. BMJ Open. 2019;9(5):e027776.
- Azar R, Laboux T, Khedjat K, Nicolazzi P, Duval M. Nutrition in daily hemodialysis. Nutr Clin Metab. 2019;33(2):122-125.
- Kansal SK, Morfin JA, Weinhandl ED. Survival and kidney transplant incidence on home versus in-center hemodialysis, following peritoneal dialysis technique failure. Perit Dial Int. 2019;39(1):25-34.
- Sriravindrarajah A, Kotwal SS, Sen S, et al. Impact of supplemental private health insurance on dialysis and outcomes. Intern Med J. 2019;May. [epub ahead of print].
- Viswanath N, Harichandra Kumar KT, Haridasan S, Parameswaran S, Priyamvada PS. Functional status in hemodialysis-a comparative study with FIM, ADLQ and 7D5L instruments. Indian J Nephrol. 2019;29(3):172-178.
- Dialysis modalities for the treatment of end-stage kidney disease [summary]. CADTH Optimal use summary tool. Ottawa (ON): CADTH; 2018: https://cadth.ca/sites/default/files/pdf/OP0526OptimalUseReport_Dialysis_postcard_tool.pdf. Accessed 2019 Aug 7.
- Chronic kidney disease. Victoria (BC): HealthLink BC; 2018: https://www.healthlinkbc.ca/health-topics/aa65427. Accessed 2019 Aug 7.
- Grainger J, Hoyer P, Milad J, Bebb C, Breen C, Day C. Assessment of the safety, efficacy and usability of a novel hemodialysis system [abstract]. Nephrol Dial Transplant. 2017;32(Suppl 3):iii624. Presented at: European Renal Association-European Dialysis and Transplant Assocation Congress; 2017 Jun; Madrid, ES.
- Bebb C, Day C, Breen C, et al. Assessment of the safety, efficacy and usability of the quanta SC+ haemodialysis system [abstract]. Hemodial Int. 2017;21(1):A29. Presented at: Annual Dialysis Conference; 2017 Mar; Long Beach, CA.
- Bebb C, Milad JE, Nagra H, Hoyer P, Gardner A. Assessment of the safety, efficacy and usability of the Quanta SC+ haemodialysis system, in-centre in 23 patients during 77 dialysis treatments [abstract]. Nephrol Dial Transplant. 2016;31(1):i492. Presented at: European Renal Association-European Dialysis and Transplant Assocation Congress; 2016 May, Vienna, AT.
- Bebb C, Day C, Breen C, et al. Implementation of a compact haemodialysis system in a real-world environment: Assessment of user uptake, safety, performance and usability [abstract]. Nephrol Dial Transplant. 2018;33(Suppl 1):i507. Presented at: European Renal Association-European Dialysis and Translpant Aassociation Congress; 2018 May; Copenhagen, DK.
- Hoyer P, Vincent C, Fantham J, et al. Human factors testing results for a novel hemodialysis system (SC+) designed for ease of use across all care settings and for operation by patients and health care professionals. Hemodial Int. 2017;21 (1):A26-A27.
- Pauly RP, Eastwood DO, Marshall MR. Patient safety in home hemodialysis: quality assurance and serious adverse events in the home setting. Hemodial Int. 2015;19(Suppl 1):S59-70.
- Wong B, Zimmerman D, Reintjes F, et al. Procedure-related serious adverse events among home hemodialysis patients: a quality assurance perspective. Am J Kidney Dis. 2014;63(2):251-258.
- Bebb C, Breen C, Day C, Mitra N, Milad J, Grainger J. Implementation of a compact haemodialysis system in a real-world environment: assessment of user uptake, safety, performance and usability [poster]. Presented at: European Renal Association-European Dialysis and Translpant Aassociation Congress; 2018 May; Copenhagen, DK.
- Dimitrijevic M, Bellovich K, Topf J, Tayeb J, Henderson H, Khairullah Q. Safety and adequacy of NxStage® home dialysis machine providing acute dialysis in a rural hospital [abstract]. Hemodial Int. 2019;23(1):A6-A7. Presented at: Annual Dialysis Conference; 2019 Mar; Dallas, TX.
- Quaranta P, Giudice D, Fersurella M, Merola G, Minonne G, Montinaro V. La comunicazione come strumento infermieristico per l'empowerment del paziente in dialisi. GTND. 2019;31(1):49-53.
PredictSURE IBD: A Whole-Blood Test Providing Long-Term Prognostic Data to Guide the Clinical Management of Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is an immune-mediated disease that causes chronic inflammation and damage to the gastrointestinal (GI) tract.1-3 IBD is a term encompassing multiple conditions; the two primary types of IBD are ulcerative colitis (UC) and Crohn disease (CD).3 Because of the complexity and relapsing nature of UC and CD, it can be difficult for those with IBD to receive a proper diagnosis and effective treatment.1,2
PredictSURE IBD is the first CE-marked, personalized medicine test for IBD. It uses gene expression profiling to guide treatment options for individuals with IBD.4 The test helps to predict the severity of CD or UC in recently diagnosed patients and may lead to early, appropriate anti‒tumour necrosis factor (anti-TNF) treatment.4
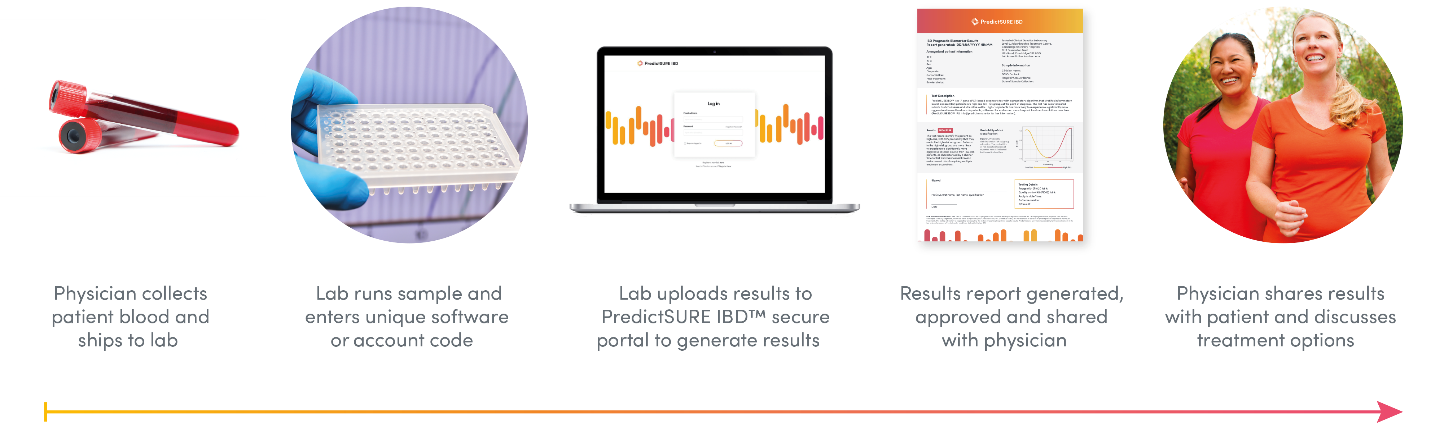
How It Works
PredictSURE IBD uses the transcription and gene expression profiling of whole blood to predict the course and disease severity of IBD in individuals with UC or CD.4 This quantitative polymerase chain reaction test stratifies the results into two subgroups, IBDhi and IBDlo, depending on whether the individual has an aggressive (high-risk) or milder (low-risk) course of IBD.5 The test measures the expression and signature — the weighted expression levels are consistent with a biological phenotype known as T-cell exhaustion — 6,7 of 17 genes of known importance for IBD and other immune-mediated diseases predictive of the risk of aggressive or mild symptoms. Knowing the likely long-term outcomes in CD or UC can help select the course of treatment most likely to be effective through personalized medicine rather than the standard course of treatment.4,5
Who Might Benefit?
A 2018 report8 by Crohn’s and Colitis Canada states that Canada has the highest prevalence of IBD cases in the world, with approximately 270,000 Canadians living with IBD: 135,000 with CD, 120,000 with UC, and 15,000 with an unclassified type of IBD. PredictSURE IBD could benefit those with IBD by indicating whether a person is more likely to experience a severe or relapsing form of either UC or CD, thus providing information that can lead to personalized treatment options, particularly regarding early therapy with biologics.4,5 People with IBD often go through a trial-and-error process with drug therapies to determine which treatment provides the best symptom relief and reduces relapse or flare-ups.4,5 Therefore, this test may reduce the time to effective treatment and improve quality of life for those with IBD. Additionally, physicians may benefit from the test, as it may provide information to help inform decision-making regarding the treatment most likely to be effective for their patients.4,5
Availability in Canada
According to the manufacturer, PredictSURE IBD received approval as an in vitro diagnostic device, is available throughout the UK, and is currently being launched in the European Union (Karen Hills, Director Medical Affairs and Marketing, PredictImmune Ltd., Cambridge, UK: personal communication, 2019 August 08). PredictImmune will announce its US partner in late 2019, leading to the test being commercially available in both the US and Canada in early 2020 (Karen Hills: personal communication, 2019 October). The manufacturer hopes to make the test available worldwide through local or regional laboratories or distributors (Karen Hills: personal communication, 2019 August).
What Does It Cost?
The price of the test in Canada has not been determined and is dependent upon local partnerships with laboratories and distributers (Karen Hills: personal communication, 2019 August). A report conducted by NICE‒National Institute for Health and Care Excellence in the UK states that the price of PredictSURE IBD is approximately £1,250 per patient.5 This does not include standard of care costs such as physician consultations or fees, follow-up fees, or drug treatment. NICE states that “the resource impact could be much lower than the current standard of care if starting anti-TNF therapy early leads to disease remission and prevents disease flare-ups but this is uncertain because it depends on the positive predictive value of the test, which is not yet determined.”5
Current Practice
Current practice does not include tests that predict the severity and course of disease for IBD. There are multiple treatment options and drug classes for UC and CD that aim to induce disease remission and relieve symptoms. Five medication classes and treatments are predominantly used for UC and CD depending on the severity of the disease: 5-aminosalicylic acid, corticosteroids, immunosuppressants, anti-TNF therapies, and other biologic treatments.9,10 Corticosteroids are often used as first-line drug therapy either for the first presentation of CD or UC, or for those who have a single inflammatory exacerbation of either CD or UC in a 12‑month period.9,10 If corticosteroids are contraindicated, 5-aminosalyclic acid and immunosuppressants are often prescribed as another first-line therapy option. Anti-TNF’s are the recommended treatment option for adults with severe active CD or UC who have not responded to first-line treatment therapy.9,10 Patients are monitored by physicians and generally try multiple drug therapies to find the correct course of treatment for their IBD if treatments fail or the disease progresses without symptom relief.9,10
What Is the Evidence?
There have been early studies11-13 examining whether gene expression and biomarkers similar to the biomarkers in PredictSURE IBD can better predict the severity of IBD in individuals and lead to new therapeutic opportunities. Results from an earlier study11 conclude that “autoimmune and inflammatory conditions may be influenced by common pathways and identifies what we believe to be the first biomarker that can predict prognosis in both UC and CD from diagnosis, a major step toward personalized therapy.” Another study13 looking at gene expression and T-cell exhaustion concluded that “T cell exhaustion plays a central role in determining outcome in autoimmune disease and targeted manipulation of this process could lead to new therapeutic opportunities.”
More recently, a study12 conducted by PredictImmune validated whether the prognostic biomarker used in the PredictSURE IBD test could stratify patients based on their long-term outcomes (high- or low-risk following an aggressive and frequent relapsing disease course), leading to personalized medicine.
Currently, there is no direct evidence to demonstrate that the use of PredictSURE IBD in conjunction with differential treatment choice (driven by a patient’s high- or low-risk status) will result in improved outcomes. However, there is an ongoing study by PredictImmune — the PROFILE study — seeking to provide concrete evidence on this.14,15 Additionally, the manufacturer is currently recruiting for another study,15 the PRECIOUS study, in the US that may recruit patients in Canada. The study will use the PredictSURE IBD test and associated biomarkers to validate whether the test works effectively in a broader population that includes people of multiple ethnicities and than that found in the original validation study, and therefore more generalizable to the patient population diagnosed with IBD.12
Issues to Consider
As the cost and distribution of PredictSURE IBD has yet to be determined in Canada, it is worth considering whether this test will be available through the public health care system or whether individuals requiring the test will need to pay out of pocket or with private insurance plans.
Related Developments
Currently, PredictSURE IBD is the only biomarker test available to better predict the severity of UC and CD. However, similar blood-based tests that aim to provide a more accurate diagnosis and personalized medicine options are available and being developed for other autoimmune conditions. For example, the company Myriad RBM16 is a certified laboratory that provides immunoassay services to analyze specific biomarkers for a variety of diseases.
Looking Ahead
PredictSURE IBD is a new and innovative technology; however, more evidence is needed to understand the effectiveness and utility of this test for the personalized treatment of IBD.
Camille Dulong
References
- Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133-146.
- Defrees DN, Bailey J. Irritable Bowel Syndrome: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Prim Care. 2017;44(4):655-671.
- GI Society: Canadian Society of Intestinal Research. The Irritable Gut 2019; https://badgut.org/information-centre/a-z-digestive-topics/the-irritable-gut-ibs-vs-ibd/. Accessed 2019 Sep 30.
- PredictImmune. PredictSURE IBD TM. 2019; https://www.predictimmune.com/predictsure-ibd-2/. Accessed 2019 Aug 28.
- National Institute for Health Care and Excellence. PredictSure-IBD for inflammatory bowel disease prognosis (Medtech innovation briefing MIB178). 2019; https://www.nice.org.uk/advice/mib178/chapter/Summary. Accessed 2019 Sep 30.
- Itadani H, Mizuarai S, Kotani H. Can systems biology understand pathway activation? Gene expression signatures as surrogate markers for understanding the complexity of pathway activation. Curr Genomics. 2008;9(5):349-360.
- Liu J, Campen A, Huang S, et al. Identification of a gene signature in cell cycle pathway for breast cancer prognosis using gene expression profiling data. BMC Med Genomics. 2008;1:39.
- Impact of Inflammatory Bowel Disease in Canada. Toronto (ON): Crohn's and Colitis Canada; 2018: https://crohnsandcolitis.ca/Crohns_and_Colitis/documents/reports/2018-Impact-Report-LR.pdf. Accessed 2019 Sep 30.
- National Institute for Health Care Excellence. Crohn’s Disease: Management (NICE guideline NG129). 2019; https://www.nice.org.uk/guidance/ng129/chapter/Recommendations. Accessed 2019 Sep 30.
- National Institute for Health and Care Excellence Ulcerative Colitis: Management (NICE guideline NG130). 2019; https://www.nice.org.uk/guidance/ng130/chapter/Recommendations-for-research. Accessed 2019 Sep 30.
- Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011;121(10):4170-4179.
- Biasci D, Lee JC, Noor NM, et al. A blood-based prognostic biomarker in IBD. Gut. 2019;68(8):1386-1395.
- McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T cell exhaustion, costimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612-616.
- Parkes M, Noor NM, Dowling F, et al. PRedicting Outcomes For Crohn's dIsease using a moLecular biomarkEr (PROFILE): protocol for a multicentre, randomised, biomarker-stratified trial. BMJ Open. 2018;8(12):e026767.
- Precious Studies. The PRECIOUS Study: Predicting Crohn's & Colitis Outcomes in the United States. 2019; https://www.preciousstudy.com/. Accessed 2019 Oct 2.
- Myriad RBM. Myriad RBM. 2019; https://myriadrbm.com/. Accessed 2019 Oct 2.