Last Updated : July 26, 2024
The Real-World Evidence Steering Committee is a collaborative initiative, chaired by CADTH, responsible for supporting the development of a pan-Canadian strategic framework (infrastructure and process) for the use of RWE in regulatory and reimbursement decision-making for drug products by publicly funded programs in Canada.
Steering Committee Membership
The Steering Committee is chaired by CADTH and Health Canada and includes the following members:
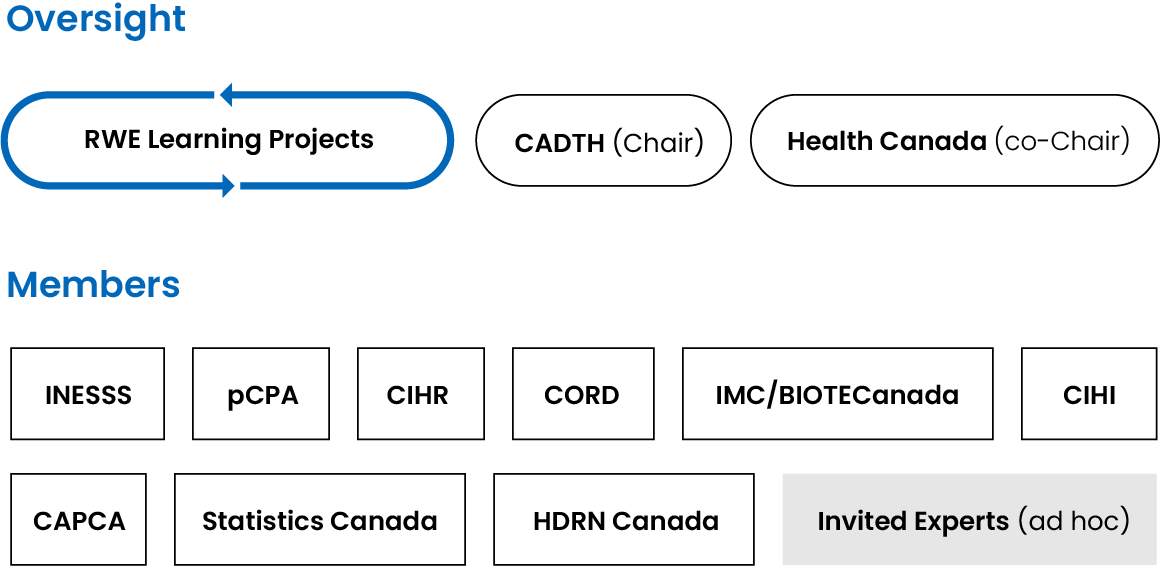
The pan-Canadian member organizations that are part of the Steering Committee may each appoint 2 representatives to the committee.
In addition, the committee will call upon expert members. These expert members will be selected based on their knowledge and expertise with respect to RWE and a particular project. Expert members may be patients, health care providers, academics, ethicists, payers, or health policy decision-makers.
Roles and Responsibilities
The roles and responsibilities of the RWE Steering Committee are to:
- guide and support the development of a strategic framework for the optimal integration of RWE into regulatory and health technology assessment (HTA) programs and health care decision-making
- provide guidance on the development of learning projects about RWE for health care decision-making
- provide guidance on the development of collaborative processes between Health Canada and other organizations involved in health care decision-making that facilitate the optimal integration of RWE
- provide oversight to the RWE Steering Committee working groups.
Meetings
The RWE Steering Committee meets a minimum of 4 times per year. A written summary of the RWE Steering Committee meetings will be posted on the CADTH website within 60 days of the meeting. Links to meeting summaries are provided below:
Last Updated : July 26, 2024

